INTRODUCTION
Malignant Peripheral Nerve Sheath Tumour (MPNST) is a term that is used to describe a tumour which originates from the peripheral nerves or their sheaths and it has replaced the old heterogeneous and confusing terminologies of a malignant schwannoma, a neurogenic sarcoma or a neurofibrosarcoma [1]. MPNSTs are rare soft tissue sarcomas which are derived from the Schwann cells or the Pleuripotant cells of the neural crest [2]. The estimated incidence of MPNSTs in the general population is 0.001%; however, it can increase from 2 to 5%-42% in patients with neurofibromatosis type 1with an aggressive course [1,2]. The peak incidences of these tumours occur around the ages of 20 to 50 years .MPNSTs originate from the peripheral nerve root trunk, extremities and the head and neck region [2]. MPNST is very rare tumour with an incidence of 1 per 1,00,000 population, which constitutes between 3 to 10 % of all the soft tissue sarcomas. Hence, this entity is often managed as a sub-category of soft tissue sarcomas [3,4].
Here, we are presenting a case of a giant, sporadic, malignant, peripheral nerve sheath tumour of the left thigh in a 65 year old female. In our case, there was no clinical evidence of neurofibromatosis type 1 and NF1 independent MPNSTs are very rare.
CASE REPORT
A 65 years old female came to the surgical OPD of our hospital with the chief complaint of a swelling in the left thigh which was there since 3-4 months. Dull aching pain over the swelling and a gradual increase in the size of the swelling were noted since the past 1 month. The patient gave a history of a difficulty in walking, sitting and squatting due to its huge size, since the past 1 to 2 months. There was no history of tingling and numbness over the left lower leg. There was no history of a sudden increase in the size of the swelling. There was no history of any major clinical illness or of hypertension, diabetes or asthma. The past history of a trauma which was caused by a blunt object to the left thigh was obtained 3 years back. There was no clinical evidence which was suggestive of neurofibromatosis-1 and there was no family history of any NF1 lesion.
On local examination, the swelling was found to be on the posteromedial aspect of the left thigh, which measured 25x20 cms in the middle one third, below the ischial tuberosity. The swelling was mobile in nature, mild and tender and it was fixed to the overlying skin, soft tissue and the muscle, but it was free from bone. The swelling was non pulsatile, with normal local temperature. On systemic examination, the CVS, RS, P/A, and the CNS were found to be within normal limits. The patient was averagely built and poorly nourished. The provisional clinical diagnosis of a neoplastic lesion with haemorrhage or a haematoma was proposed. The laboratory results which included the blood count, urine analysis and chest X-ray/USG of the abdomen were all unremarkable. Computerized tomography and MRI were suggestive of a 20x18 cm soft tissue mass which was free from bone and joints s/o a mesenchymal neoplasm. Both the kidneys and the liver were unremarkable. FNAC of the lesion was advised, which was suggestive of a spindle cell tumour (intermediate grade sarcoma-? liposacoma, ? fibrosarcoma) and a biopsy of the lesion was advised. The patient underwent a wide local excision of the mass, with wide surgical margins resection. The mass (18x16 cms) was solid and cystic in nature and it was adherent to the sciatic nerve. By carefully separating it from the sciatic nerve, the mass was excised and it was sent for histopathological studies. The post operative period was uneventful.
GROSS FINDING
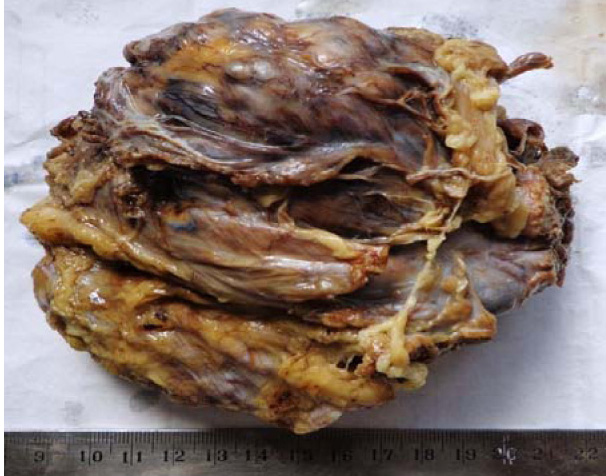
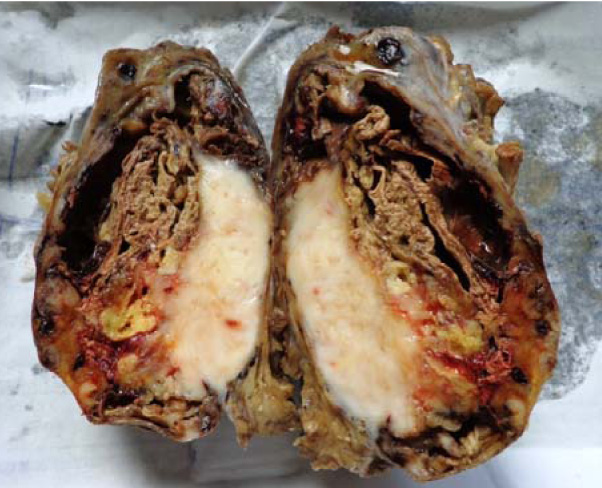
We received the excised mass of the left thigh, which totally measured 14x13x7cms and weighed 480 gms. Its external surface was well circumscribed, nodular and irregular, with congested blood vessels [Table/Fig-1]. On cutting it open, a grey white mass with a variegated appearance, with an area of a haemorrhage, necrosis and cystic changes, was noted [Table/Fig-2]. The grey white fleshy mass measured 9x6 cms. The cystic area with foci of necrosis was also noted.
External surface of excised mass (left thigh) was well circumscribed, nodular and irregular with congested blood vessels
Cut section showed grey white mass with variegated appearance with area of hemorrhage, necrosis and cystic change
LIGHT MICROSCOPY
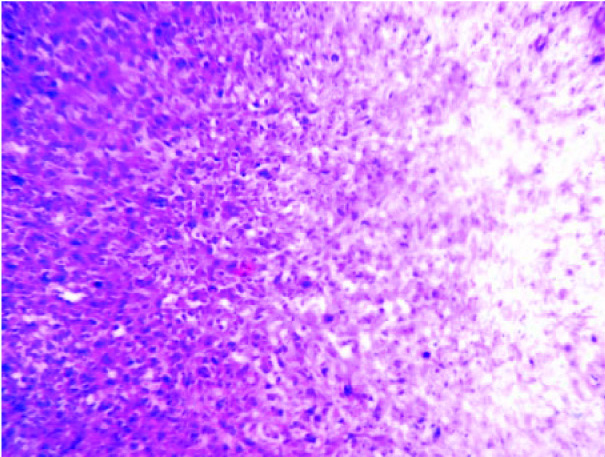
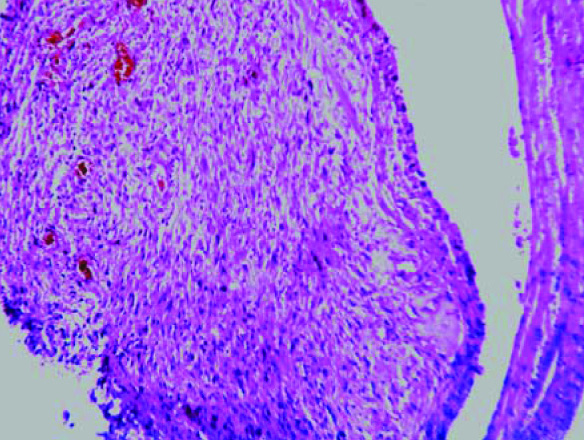
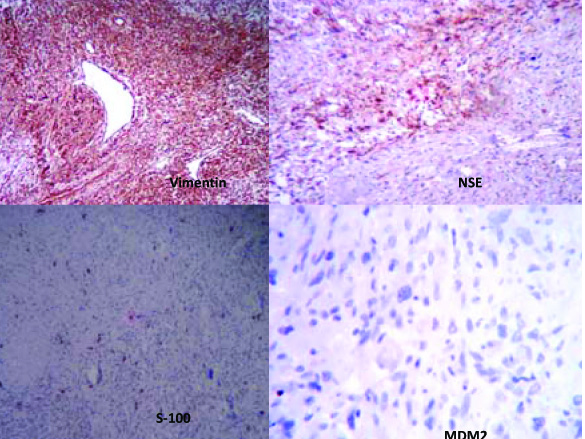
Multiple sections which were studied, showed a well circumscribed tumour with a biphasic pattern, with hypercellular and hypocellular areas [Table/Fig-3]. The hypercellular areas were composed of tumour cells which were arranged in fascicles, sheets, storiform patterns and nodules [Table/Fig-4]. The individual tumour cells were spindle shaped, with wavy nuclei and buckling [Table/Fig-5]. Few atypical spindle cells with giant nuclei and giant forms were also noted. The mitotic cells were 2-4/hpf. The hypo-cellular areas were composed of large foci of necrosis with a classical palisading appearance and with myxoid areas. Focal areas of haemorrhage and necrosis were noted. Multiple nerves like whorls were also seen [Table/Fig-6]. The underlying muscle was free from tumour. A final histopathological diagnosis of a malignant soft tissue tumour- a malignant peripheral nerve sheet tumour was given. The patient was free from the residual tumour / recurrence for the past one year after the follow up. The immunohistochemical study confirmed the diagnosis of MPNST in view of the diffuse positivity of Vimentin, NSE with focal positivity for S-100 and negativity for MDM2 [Table/Fig-7]. Thus, the differential diagnosis of a dedifferentiated liposarcoma was ruled out.
Light microscopy showed well circumscribed tumor with biphasic pattern as hypercellular and hypocellular area (H &E stain, x100)
The hypercellular areas composed of tumor cell arranged in fascicles, sheets, storiform pattern and nodules with hypocellular areas of myxoid and necrosiswith characteristic palisading appearance (H &E stain, x100)
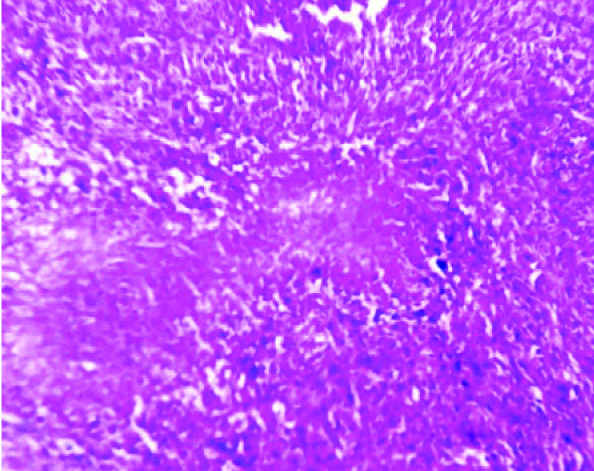
Individual tumor cells were spindle shaped with wavy nuclei and buckling. Few atypical spindle cells with giant nuclei were also noted (H &E stain, x400)
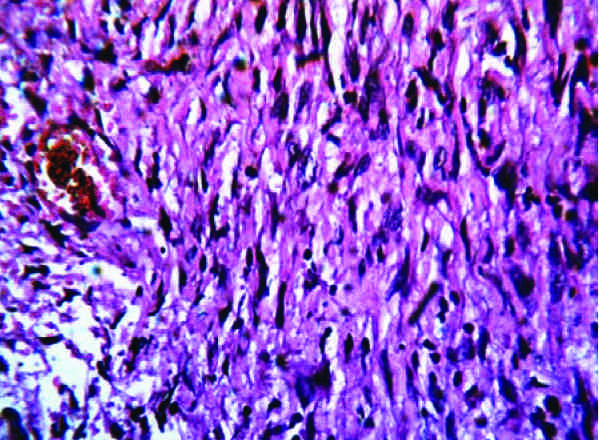
Many nerves like whorls were noted.(H &E stain, x400)
The tumor cells show diffuse immunoreactivity for Vimentin, NSE with focal positive for S-100 and negative for MDM2
DISCUSSION
A Malignant Peripheral Nerve Sheath Tumour (MPNST) is an uncommon neoplasm and a rare variety of a soft tissue sarcoma of ectomesenchymal origin [3,4]. It arises from the major or minor peripheral nerve branches or the sheaths of the peripheral nerve fibres [5,6]. This tumour may arise spontaneously in adult patients, although 5% to 42% of the MPNSTs have an association with multiple NF1s [3,7]. Thus, a combination of the gross, histopathological and immunohistological studies is used for diagnosing these tumours [3].
MPNSTs are often associated with NF1s and these are aggressive tumours that pose significant diagnostic and therapeutic challenges. About 10% of the NF1 patients may develop MPNSTs which exhibit poor prognoses [8]. The pathogenesis of MPNSTs has been poorly understood due to their complex histopathologies, but a biallelic NF1 gene inactivation is essential for the tumour development [8].Laskinet et al., [9] pointed out irradiation of the peripheral nerve as one of the possible aetiologies. Such tumours often develop after a long latent period of 10-20 years following irradiation and they account for 11% of the MPNSTs [9].
Our case, the 65 years old female was a housewife with no history of exposure to irradiation and without any clinical evidence of a NF1 (a multiple neurofibroma, Café au lait spots, congenital malformations, or other tumours, etc) in the patient or in the family. Hence, our case was of a giant, sporadic, non NF1or NF1 independent MPNST of the left leg with a favourable prognosis. Complete surgical excision is a mainstay of the treatment and a strong predictor of the survival in spite of its biological aggressive nature [10]. In our case, local excision of tumour with wide resection margins was carried out. The patient is free of symptoms after one year of follow up.
The histological diagnostic criteria for MPNSTs are as follows:[3]
A gross fusiform tumour with respect to the nerve (nerve like whorls).
The microscopic features of spindle cells with fascicular patterns and varying degrees of mitosis, necrosis and tumour calcification.
The presence of an associated benign neurofibroma or schwann cells.
A positive immunohistochemical staining for S-100,vimentin and neuron specific enolase(NSE).
The tumours are classified as low and high grade on the basis of their cellular differentiations, mitotic counts and tumour necrosis [11]. More than 50% necrosis with more than 5 mitotic figures per 10 hpf has been considered as suggestive of a high grade tumour [3]. In our case, the MPNST was of low grade in view of the 1-3 mitotic figures with 10-20% of tumour necrosis.
These tumours often create diagnostic difficulties because of their cellular origins and histopathological similarities with those of other spindle cell sarcomas. The differential diagnosis of this tumour is monophasic synovial sarcoma, dedifferentiated liposarcoma, leiomyosarcoma and fibrosarcoma [12]. The varied characteristic morphology of the tumour with the specific diagnostic patterns, help in the diagnoses of the above mentioned tumours and the diagnoses can be confirmed by immunohistopathological studies.
Mantripragada KK et al., [13] reported the first comprehensive investigation of the DNA copy number across a multitude of genes of NF1 tumours by using high resolution array Comparative Genomic Hybridization (CGH). They found specific alterations in the form of amplifications of the ITGB4, PDGFRA, MET, TP73 and the HGF genes in the pathogenesis of MPNSTs and concluded that array CGH was a novel diagnostic marker.
Real time quantitative RT-PCR was used by Levy P el al., [14] for the molecular profiling of MPNSTs by the mRNA expression of 489 selected genes in pooled MPNST samples, in comparison with pooled neurofibroma samples. They stated that RT-PCR was a promising alternative to the cDNA microarrays for the molecular tumour profiling, being more precise, reproducible, quantitative and requiring small amounts of RNA (small tumours or microdissected samples).
In 2006, Miller SJ et al., [15] found that the MPNST lines were heterogeneous in their in vitro growth rates and that they exhibited diverse alterations in the expressions of the pRb, p53, p14(Arf) and the p16(INK4a) proteins.All the MPNST cell lines expressed the epidermal growth factor receptor and they lacked the S100 beta protein. They found that the Schwann cell differentiation markers (SOX10, CNP, PMP22 and NGFR) were downregulated in the MPNSTs, whereas the neural crest markers, SOX9 and TWIST1 were overexpressed. They concluded that gene expression profiling was a potential biomarker and/ or a therapeutic target for the treatment of MPNSTs and that it could be used as a primary analytical tool [15].
In the present case, a morphodiagnosis of an MPNST was made with the help of the hypo and hyper cellular areas with the characteristic nerve like whorls of the tumour cells, fascicles and the nuclear criteria of the tumour cells. The mitotic figures and the necrosis of the tumour favoured its low grade nature and the size of the tumour suggested its giant form. There was no family history or any clinical evidence of a NF-1.So, a final histopathological diagnosis of a giant sporadic low grade MPNST of the left thigh was made. The above findings were confirmed on immunohistopathological studies, as S-100, NSE and Vimentin were positive. The patient has been on regular follow up for the past one year and is free of any residual or recurrent tumour.
CONCLUSION
In the present case, we have highlighted the sporadic nature and the giant forms of MPNSTs, which are rare clinical entities, as most of the common MPNSTs present with NF-1s.Due to their non NF-1 or NF independent nature, they have good prognoses. A combination of clinical, pathological and immunohistochemical studies help in the diagnoses of such rare tumours. Molecular diagnostic (tumour profiling) methods as RTPCR and CGH microarrays may be useful as novel diagnostic markers and therapeutic targets for the treatment of MPNSTs in the modern era.
[1]. Chalkoo M, Ahangar S, Laharwal AR, Patloo AM, Mohd A, Dar SA, Primary malignant peripheral nerve sheath tumor of breast-a case reportSurgical Science 2011 2:137-39. [Google Scholar]
[2]. Muacevic A, Wowra B, Malignant peripheral nerve sheath tumor. Case Study. Europeancyberkniefcenter, Munich, Germany. Available from www.abmedia.it/malignant_peripheral_nerve_sheth_tumour.pdf cited on 1/12/12 [Google Scholar]
[3]. Kar M, Deo SVS, Shukla WK, Malik A, Gupta SD, Mohanty BK, Malignant peripheral nerve sheath .Clinicopathological study and treatment outcome of 24 casesWorld Journal of Surgical Oncology 2006 4:55 [Google Scholar]
[4]. Hruban RH, Shiu MH, Senie RT, Woodruff JM, Malignant peripheral nerve sheath tumor of buttock and lower extremityA study of 43 cases 1990 60:1253-65. [Google Scholar]
[5]. Cashen DV, Parisien RC, Raskin K, Hornicek FS, Gebhardt MC, Mankin HJ, Survival data after patient with malignant schwannomaClin Orthp Relat Res 2004 426:69-73. [Google Scholar]
[6]. Hirose T, Scheithauer BW, Saro T, Peripheral malignant nerve sheath tumor a Clinicopathological, immunohistochemical and ultrastructural study of seven casesAM J Surgical Pathology 1998 22:1368-78. [Google Scholar]
[7]. Erans DG, Baser ME, McGaughram J, Sherif S, Howard E, Moran A, Malignant peripheral malignant nerve sheath tumor in NF-1J Med Genet 2002 39:311-14. [Google Scholar]
[8]. Upadhyaya M, Genetic basis of tumourogenesis in NF1. Malignant peripheral malignant nerve sheath tumorFront Biosci 2011 1(16):937-51. [Google Scholar]
[9]. Laskim WB, Silvermann TA, Enzinger PM, Post radiation soft tissue sarcoma. An anylasis of 53 casesCancer 1988 62(11):2330-40. [Google Scholar]
[10]. Friedrich RE, Hartmann N, Mautner VF, Malignant peripheral nerve sheath tumor MPNST in NF1 affected childrenAnticancer Research 2007 27:1957-60. [Google Scholar]
[11]. Trojani M, Contesso G, Coindre JM, Ruwsse J, Bui NB, Soft tissue sarcoma in adults. Study of pathological prognostic variable and definition of histological grading systemInternational J cancer 1984 33:37-42. [Google Scholar]
[12]. Ducatman SB, Bernd WS, David GP, Herbert MR, Duare MI, Malignant peripheral malignant nerve sheath tumorA clinicopathological study of 120 cases 1986 57:2006-12. [Google Scholar]
[13]. Mantripragada KK, Spurlock G, Kluwe L, Chuzhanova N, Ferner RE, Frayling IM, High resolution DNA copy number profiling of malignant peripheral nerve sheath tumor using targeted microarray base comparative genomic hybridizationClin Cancer Res 2008 14:1015-24. [Google Scholar]
[14]. Levy P, Viaud D, Leroy K, Laurendeau I, Wechsler J, Bolasco G, Molecular profiling of malignant peripheral nerve sheath tumor associated with NF 1, based on large scale real time RT-PCRMolecular Cancer 2004 3:1-13. [Google Scholar]
[15]. Miller SJ, Rangwala F, Williams J, Ackerman P, Kong S, Jegga AG, Large scale molecular comparison of human schwann cells to malignant peripheral nerve sheath tumor cell lines and tissuesCancer Res 2006 66:2584-91. [Google Scholar]