Introduction: Radiation is increasingly being used for medical purposes and it is an established weapon in the diagnosis and the therapy of cancer. An exposure to 1-2 Gys causes the NVD (Nausea, vomiting, diarrhoea) syndrome, whereas an exposure to 2-6 Gys causes the haematopoietic syndrome. The aim of the present study was to investigate the protective effect of the Nardostachys jatamansi root extract (NJE) on the radiation induced haematological damage in rats.
Materials and Methods: EBR was performed at the Microtron Centre, Mangalore University, India. Rats were treated with NJE once daily for 15 days before and after the irradiation. After the irradiation, blood was collected for determining the peripheral blood counts (RBC and WBC), haemoglobin, the platelet count and the packed cell volume (PCV) at 6 hours, 12 hours, 24 hours, 48 hours and 5, 10 and 15 days post irradiation. The data was analyzed by one way ANOVA, followed by the Tukey’s test for multiple comparisons.
Result: NJE provided protection against the radiation induced haematological disorders. The rats treated with NJE exhibited a time dependent significant elevation in all the haematological parameters which were studied and its modulation upto the near normal level was recorded.
Conclusion: From this study, we concluded that, NJE provides protection by modulating the radiation induced damage on the haematopoietic system.
INTRODUCTION
The widespread use of radiation in the diagnosis, industry and the energy sector and its inadvertent exposure during air and space travel, nuclear accidents and nuclear terror attacks requires a safeguard against human exposures [1]. An exposure to ionizing radiation increases the production of the reactive oxygen species (ROS) and this can lead the irradiated cells into a state of oxidative stress, which has been implicated in an enormous variety of natural and pathological processes [2]. Hence, a pharmacological intervention could be the most prudent strategy which can be used to protect humans against the harmful effects of ionizing radiations. Radiation is increasingly being used for medical and occupational purposes and it is an established weapon in the diagnosis and the therapy of cancer. Radiation therapy injures or destroys the cells in the areas which are being treated ("target tissues") by damaging their genetic materials. So, the amounts of ionizing radiation that can be given to treat malignant tumours are limited.
Radio therapy is the medical use of ionizing radiations as a part of the cancer treatment to control malignant cells. Total body irradiation (TBI) is a radiotherapy technique which is used to prepare the body for receiving a bone marrow transplant. It has been reported that low-energy electron beams are advantageous because of their depth dose characteristics, although they have a rather limited place in the radio therapeutic practice [3]. Radiotherapy actually kills the highly proliferate cells, but it can damage other normal cells also, in each therapeutic process. An exposure to 1-2 Gys causes the NVD (Nausea, vomiting, diarrhoea) syndrome and an exposure to 2-6 Gys causes the haematopoietic syndrome in which the bone marrow, spleen and the thymus get affected. An exposure to 8-15 Gys causes the gastrointestinal (GI) syndrome, whereas an exposure to >25 Gys causes the Central Nervous System (CNS) syndrome [2]. The approximate time of death varies between 30 days to 48 hours, depending on the dosage and the time of exposure [2]. In recent years, radiaoprotective agents with novel modes of action have been under investigation; in particular, the compounds that can affect the haematopoietic stem cell regeneration have attracted significant interest. The aim of this strategy was to increase the survival rate by stimulating the function and the regeneration of the stem cell population that has become decreased, due to radiation induced damage.
The traditional Indian system of medicine, Ayurveda, gives a detailed account of several diseases and their treatments. The majority of drugs and/or drug formulations which are used in Ayurveda are principally derived from herbs and plants. Plant extracts such as Garlic, Ginsing, Aloe vera, Podophyllum, Ocimum, Spirulina, Mentha piperita and various herbal drug preparations have been found to have protective effects against the radiation induced disorders in mammals [3–11]. In other studies which were done on Citrus extracts, the Hawthrown fruit extract, the Abana extract [12], diltiazem [13], hesperidin [14], the extract of Amaranthus paniculatus, Liv-52 [15], the methanolic extract of the Grewia asiatica fruit, Triphala [16], Curcuma longa (Curcumin-Di-feruloyl-methane and curcuminoids) and the extracts of Terminalia arjuna, Terminalia chebula and Wagatia spicata have also demonstrated radio protective activities [17].
Nardostachys jatamansi, a medicinal herb which belongs to the family, Velirenaceace of the plant taxa, has been extensively described in the Ayurvedic literature. The bitter tonic which is obtained from the rhizomes of N. Jatamansi (Bhutajata, Jathilaa, Thapaswini) can be used as a neuroprotective, sunscreen, stimulant, antispasmodic, repellent, antipyretic and antioxidant, as well as to treat the Herpes infection, leprosy, various neuropsychiatric illnesses, and excessive thirst [18, 19]. Some plants like Santalum album [20], Embelia ribes [21], Piper longum, Zinger officinale, Syzygium aromaticum, Elettaria cardamum [22], Rubia cordifolia [23], Ocimum sanctum [24], Asparagus racemosus, Glycorrhiza glabra, Phyllanthus embelica, Boerrhaavia diffusa and Eclipta alba have been found to possess antioxidant properties [25] and some of them have proved to be radioprotective.
The root extract of N. Jatamansi is aromatic, antispasmodic, diuretic, emmenagogue, neuro protective, a tonic, a carminative, a de obstruent, a digestive stimulant, and a reproductive. It has other Ayurvedic applications, such as in the complexion, strength, kidney stones, jaundice, in the removal of blood impurities, spasmodic hysteria, other nervous convulsive ailments, heart palpitations, nervous headache, flatulence, epilepsy, convulsions, respiratory and digestive diseases, skin diseases, typhoid, gastric disorders and seminal debility. Since N. Jatamansi meets few of the characteristics of the radiaoprotective agents, we hypothesized that irradiated rats which were treated with NJE would exhibit a better protection with respect to the haematopoietic system as compared to the irradiated rats without the NJE administration. This prompted us to evaluate the radiaoprotective effect of the Nardostachys jatamansi root extract (NJE) on the radiation induced haematological alterations.
MATERIAL AND METHODS
Chemicals
Ethylene Diamine Trichloroacetic Acid (EDTA), Ferric chloride, sodium bicarbonate, hydrochloric acid (HCl), sodium chloride, potassium hydrogen phosphate, disodium hydrogen phosphate, potassium chloride and hydrogen peroxide were procured from Ranbaxy Fine Chemicals, New Delhi, India.
The composition of Nardostachys jatamansi
The roots of this plant contain an essential oil which is rich in sesquiterpenes and coumarins. Jatamansone or Valeranone is the principal sesquiterpene. The other sesquiterpenes include Nardostachone, Dihydrojatamansin, Jatamansinol, Jatamansic Acid, Jatamansinone, Jatamansinol, Oroseolol, Oroselone, Seselin, Valeranal, Nardostachyin, Nardosinone, Spirojatamol, Jatamol A and B, Calarenol, Seychellene, Seychelane, Coumarin: Jatamansinor Xanthogalin. A new sesquiterpene acid, Nardin and new pyranocoumarin: 2′, 2′-Dimethyl-3′-Methoxy-3′, 4′-Dihydropyranocoumarin and Actinidine, which is an alkaloid, have also been reported [26].
The animal care and handling
The animal care and handling were done according to the guidelines which were set by the World Health Organization (WHO) and the National Research Council’s guidance for the care and use of laboratory animals. Male Albino Wistar rats which were aged 2 months, with 150±20 gm body weight were used in the present study, after getting the approval of the institutional ethical committee for the protocol. The animals were fed with the standard rat feed, with free access to water ad libitum and they were maintained under well ventilated conditions of 12 hour light and dark cycles at a temperature of 24-28°C. They were kept in standard polypropylene cages with husk shavings as bedding and were adapted to the laboratory conditions for 7 days prior to the whole body electron beam irradiation. The animals were randomly assigned to cages (n=6 in each group) and the individual animals were fur marked with blue ink for an easy identification. All the experiments were performed during the same time of the day.
Preparation of the extract and its mode of administration
An extract of the N. jatamansi root was prepared by extracting 100 grams of Jatamansi powder (Indian Remedies, India) in 90% ethanol (1 Ltr) at 50°C to 60°C in a Soxhlet extractor for 72 hours. The cooled liquid extract was concentrated by evaporating its liquid contents in a rotary evaporator and an approximate yield of 20% was obtained. The dried ethanol extract was suspended in distilled water. The drug, NJE, was administered orally, once daily at the dosage of 400mg/kg bw, for 15 consecutive days before the irradiation, after the irradiation and both before and after the irradiation.
The experimental design
In the present study, thirty six male Albino Wistar rats were used and they were randomly divided into six groups of six animals each and the individual animals were fur marked with Indian ink (blue) for an easy identification.
The animals were divided as follows
Group-A (Control) were fed with a normal diet and double distilled water.
Group-B (Radiation Control) were exposed to 3Gys of whole body electron beam irradiation.
Group-C (Drug Control) were treated orally with an aqueous suspension of NJE, 400 mg/kg bw.
Group-D (Pre-irradiation treatment group) were treated orally with an aqueous Suspension of NJE, 400 mg/kg bw before the irradiation.
Group-E (Post-irradiation treatment group) were treated orally with an aqueous suspension of NJE, 400 mg/kg bw days after the irradiation.
Group-F (Pre and Post-irradiation treatment group) were treated orally with an aqueous suspension of NJE, 400 mg/kg bw before and after the irradiation.
The irradiation
Whole body electron beam irradiation (EBR) was done at the Microtron Centre, Mangalore University, at a dose of 3Gys with n=6 animals in each group. The unanaesthetized animals were restrained in well-ventilated Plexiglas sheet containers with perforations and they were exposed to EBR at a distance of 30 cm from the beam exit point of the Microtron accelerator, at a dose rate of ~100 Gys/min at room temperature (23±2°C). The animals of all the groups were monitored daily for the development of the symptoms of radiation sickness and mortality.
Haematological Parameters
Blood was collected from the tail vein of each animal in a vial which contained 0.5M EDTA. The peripheral blood counts (RBC, WBC), haemoglobin (Hb), the platelet count and the packed cell volume (PCV) were determined at 6 hours, 12 hours, 24 hours, 48 hours and 5, 10, and 15 days post-irradiation by using an automated haematology analyzer (Sysmex Co., F-820, Japan).
The endogenous spleen colony forming unit (CFU) assay
For the endogenous CFU assay, the rats were sacrificed by doing cervical dislocations at the end of the experiment. The spleens were removed from all the experimental animals and they were fixed in Bouin’s fluid for 24 hours. The colonies stood out as yellow nodules against the darker background of the splenic tissue; these colonies were counted and the average number of colonies per spleen was determined. The fixation in Bouin’s solution makes the colonies more distinct and it also facilitates the counting [27].
The splenic index
The splenic index was determined from the weights of the spleens of the surviving rats. On the 15th day of the post-irradiation treatment, the animals were sacrificed and the spleens were recovered and weighed. The results were expressed as the organ indices by using the formula: weight of the spleen (mg)/body wt (g) [28].
STATISTICAL ANALYSIS
The statistical analysis of the data for the significant variations within the groups was performed by using the SPSS statistical software. It was done by using the one way analysis of variance (ANOVA) and multiple group comparisons were made by using Tukey’s HSD test. The values were expressed as mean ± S.D for the six samples in each group. A p value of < 0.05 was considered as significant.
RESULTS
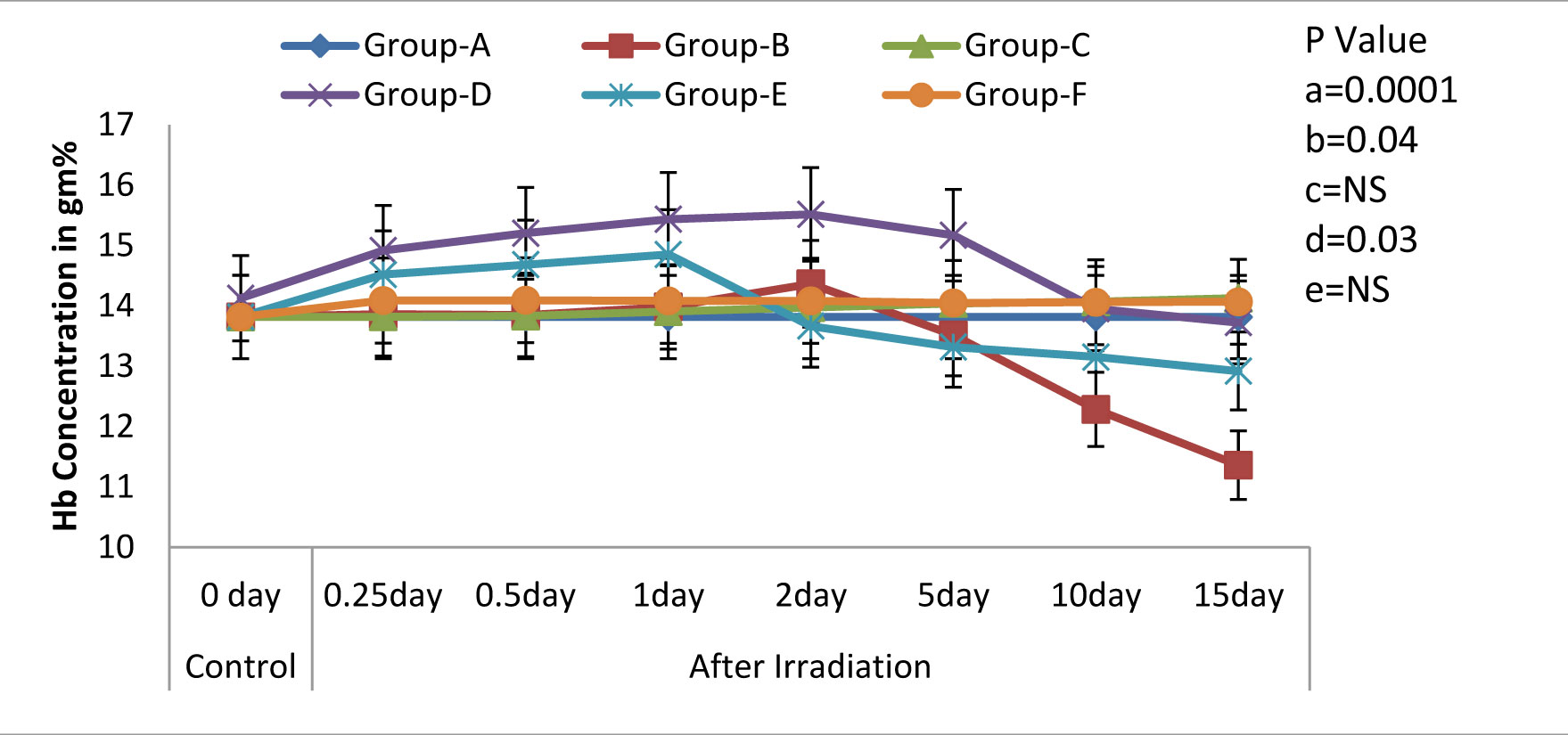
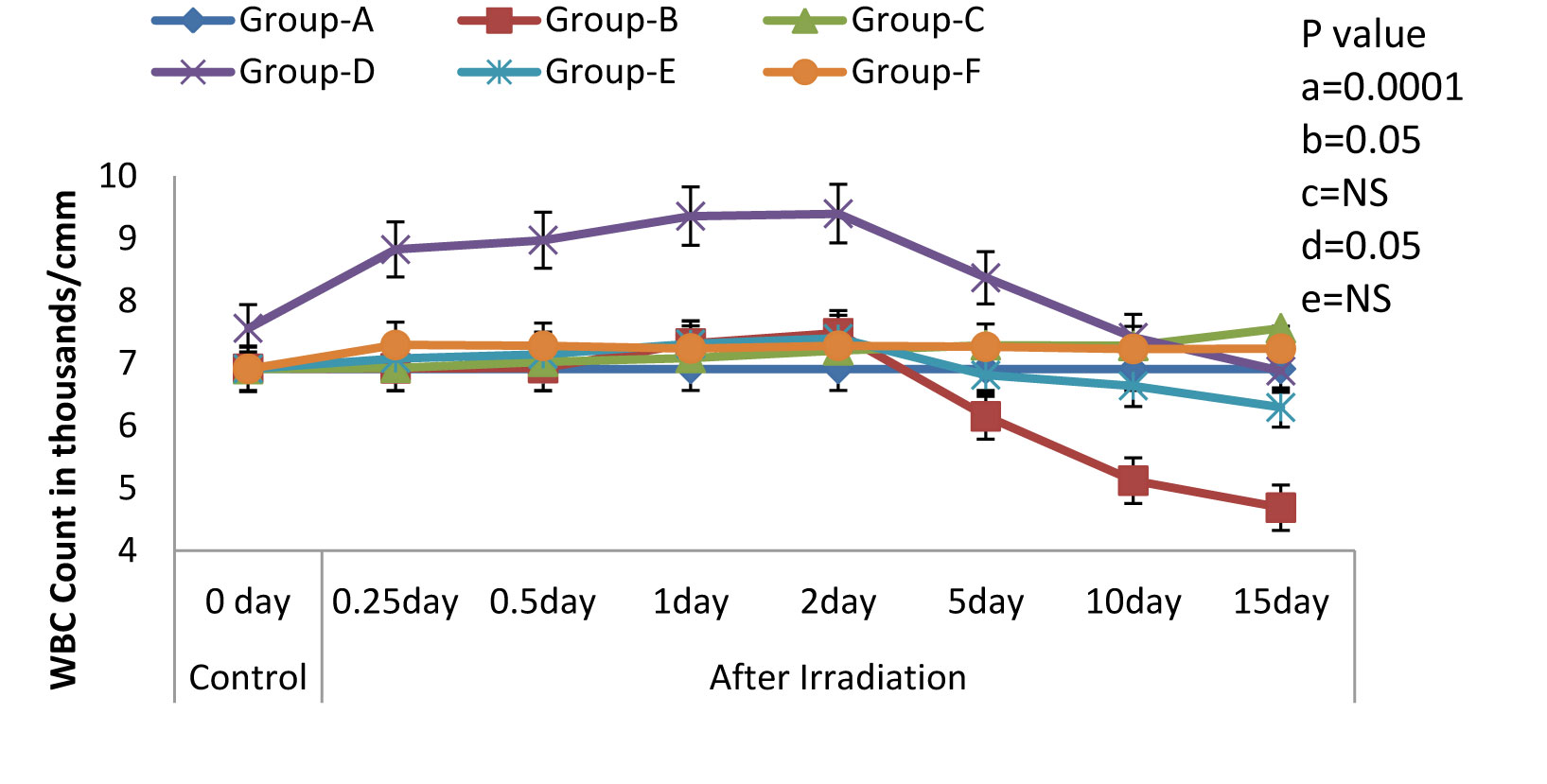
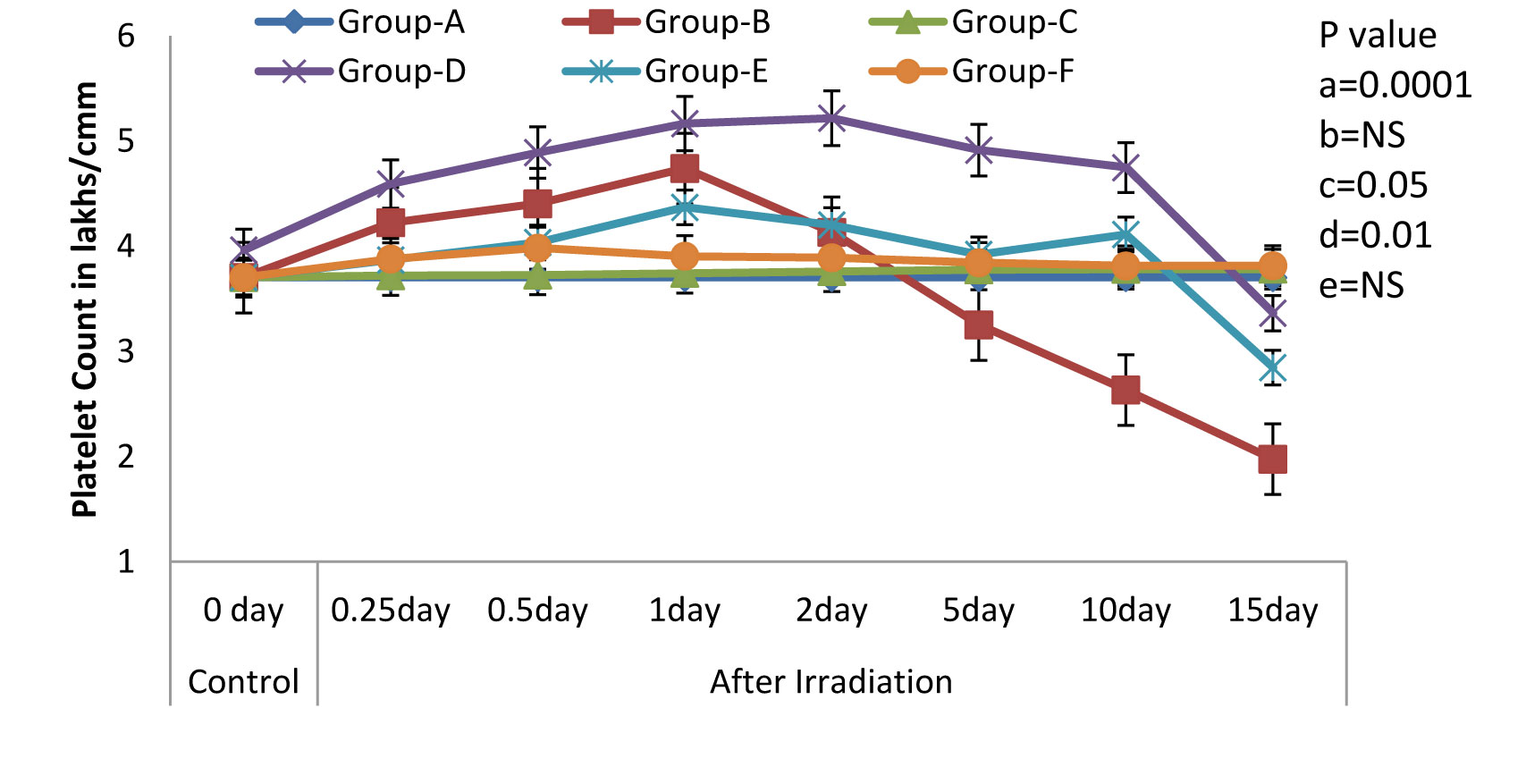
One of the most characteristic manifestations of whole body irradiation are the Haematological Syndromes. These syndromes, as a rule, are produced only by total-body or nearly total-body irradiations. In the present study, we found that the treatment with NJE provided protection against the whole body EBR induced haematological disorders in Albino Wistar rats, as was evidenced by the following results. The Hb level, the peripheral blood counts (including the RBC and the WBC counts), the platelet count and the packed cell volume in the irradiated animals (Group-B) exhibited a significant decline (p=0.0001) as compared to those in the non irradiated control group (Group-A). Whereas, a significant modulation was recorded in Groups-D and F [Table/Fig-1,2,3,4&5] that is, in the animals which were treated orally with an aqueous suspension of NJE, 400mg/kg bw respectively, once daily for 15 consecutive days before and 15 consecutive days both before and after the irradiation.
Variations (Mean ± S.D.) in the level of Haemoglobin in rats exposed to 3Gy WBE irradiation and treated with NJE before, after, both before and after irradiation. a=Control vs. Radiation control, b=Control vs. Drug control, c= Control vs. Preirradiation treatment d= Control vs. Postirradiation treatment and e= Control vs. Pre & Postirradiation treatment groups
Variations (Mean ± S.D.) in the RBC Count in rats exposed to 3Gy WBE irradiation and treated with NJE before, after, both before and after irradiation. a=Control vs. Radiation control, b=Control vs. Drug control, c= Control vs. Preirradiation treatment d= Control vs. Postirradiation treatment and e= Control vs. Pre & Postirradiation treatment groups

Variations (Mean ± S.D.) in the WBC Count in rats exposed to 3Gy WBE irradiation and treated with NJE before, after, both before and after irradiation. a=Control vs. Radiation control, b=Control vs. Drug control, c= Control vs. Preirradiation treatment d= Control vs. Postirradiation treatment and e= Control vs. Pre & Postirradiation treatment groups
Variations (Mean ± S.D.) in the Platelet Count in rats exposed to 3Gy WBE irradiation and treated with NJE before, after, both before and after irradiation. a=Control vs. Radiation control, b=Control vs. Drug control, c= Control vs. Preirradiation treatment d= Control vs. Postirradiation treatment and e= Control vs. Pre & Postirradiation treatment groups
Variations (Mean ± S.D.) in the level of PCV in rats exposed to 3Gy WBE irradiation and treated with NJE before, after, both before and after irradiation. a=Control vs. Radiation control, b=Control vs. Drug control, c= Control vs. Preirradiation treatment d= Control vs. Postirradiation treatment and e= Control vs. Pre & Postirradiation treatment groups.
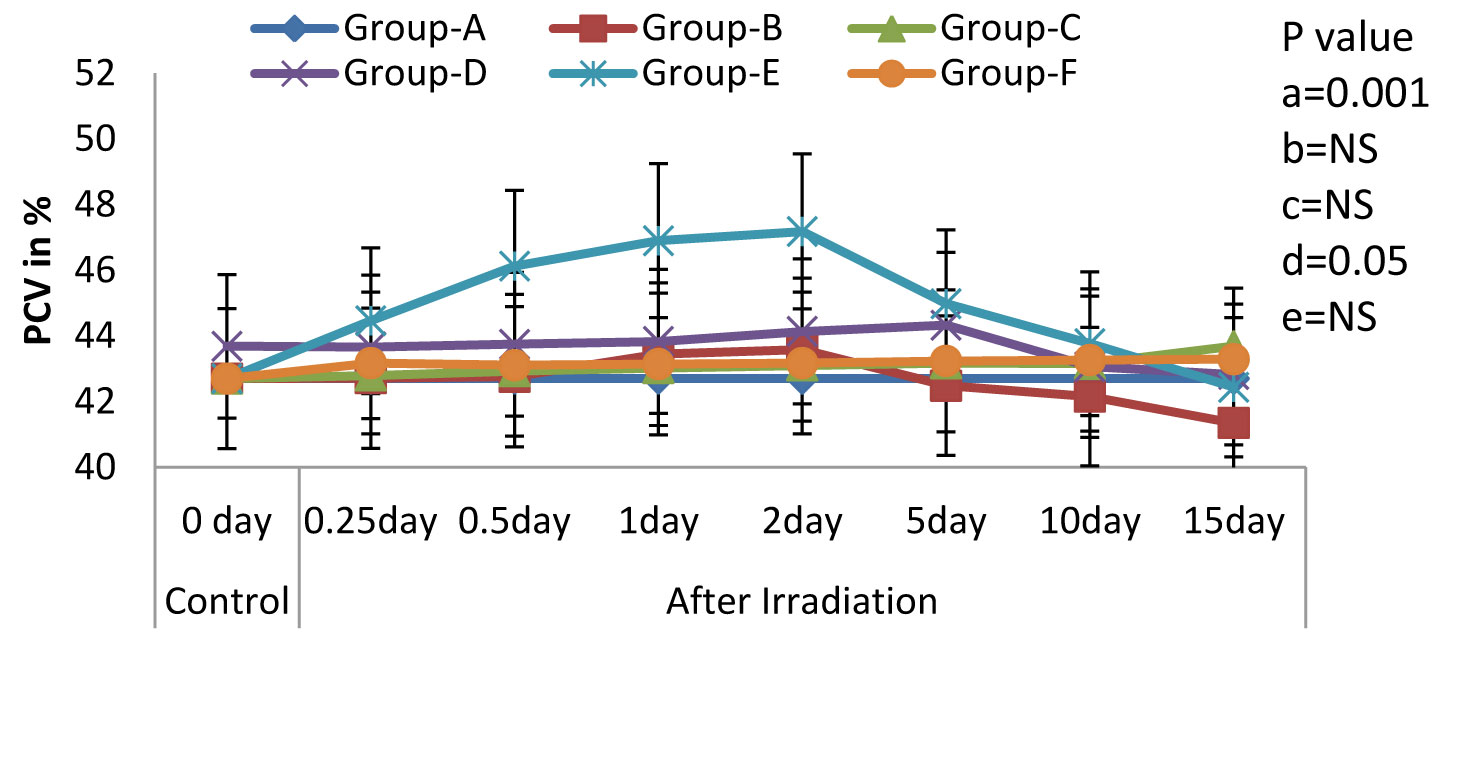
All the haematological parameters showed a progressive increase upto the 2nd post irradiation day, following a progressive decline, till the end of the experiment, in the irradiated animals. The animals which were treated with an aqueous suspension of NJE showed a significant increase as compared to the control animals and could able to increase upto near normal in Group-D and quite beyond the normal level in Group-E in all the haematological parameters. The endogenous spleen colony count and the splenic index form valuable tools in the demonstration of the repopulation of the entire haematopoietic organs in irradiated individuals. In the present study [Table/Fig-6], no colonies were observed in the control rats. But, on irradiation, the appearance of gross nodules in the spleen was evident. The spleen colony count and the splenic index were significantly and effectively modulated by the treatment, before and after the irradiation treatment.
Comparison of Endogenous Spleen Colony counts and splenic index in different experimental groups.
| Groups | Colony Counts/spleen | Splenic index |
|---|
| Control | 0 ± 0 | 0.21 ± 0.08 |
| Radiation Control | 10.0 ± 0.1*** | 0.12 ± 0.08*** |
| Drug Control | 1.3 ± 0.02(NS) | 0.24 ± 0.04(NS) |
| Preirradiation Control | 19 ± 1.45*** | 0.15 ± 0.06*** |
| Postirradiation Control | 14 ± 1.36*** | 0.13 ± 0.01*** |
| Pre & postirradiation Control | 19 ± 2.58*** | 0.21 ± 0.02(NS) |
Note: P value, NS=Non significant, * = 0.05, ** = 0.01, *** = 0.001 between control and experimental groups.
DISCUSSION
The exposure of animals to ionizing radiations causes a series of physiological changes which are known as the acute radiation syndrome, which is dependent on the exposure dose and which may even lead to death. The damage to the haematopoietic system is a major factor in the mortality, following an acute radiation exposure [29], which might be due to the fact that the proliferating cells are highly sensitive to irradiations. In the present study, there was a significant decrease in the levels of the haematological variables (haemoglobin, erythrocytes, leukocytes, the packed cell volume and the platelet count) in the irradiated animals as compared to those in the normal or control animals. Therefore, the effect of the whole body irradiation is mainly felt by the highly proliferating bone marrow progenitor cells. Since the bone marrow progenitor cells are crucial for life, any damage to these cells can impair the normal physiological processes, thus causing an irreversible effect on the survival of an individual. However, a significant rise in these parameters was observed in the NJE treated animals. The treatment of irradiated rats with NJE reduced the symptoms of radiation sickness and mortality also. This clearly indicated the effectiveness of N. Jatamansi in arresting the mortality which was induced by whole body EBR. The administration of NJE resulted in the protection of the rats which were exposed to the whole body EBR. This was evidenced by the significant increase in the RBC Count, the Hb concentration, the WBC Count, the platelet count and the packed cell volume, which were highly reduced in the irradiated rats. This proved that the treatment of the Albino Wistar rats with NJE significantly modulated the bone marrow depression. This proved the immunomodulating property of NJE, because, our data indicated that the stimulatory effect was not only limited to the erythropoiesis, but that it also extended to the leukopoiesis and megakaryopoiesis. This may be due to the presence of constituents that could interact and stimulate the formation and the secretion of erythropoietin and the haematopoietic growth factors/committed stem cells [30]. Our results are concurrent with the findings of the studies which were done on other plant extracts, which have been reported elsewhere [31–37].
Therefore, the treatment with NJE before and after the irradiation relatively reduced the radiation induced sickness and the mortality in rats by protecting against the radiation-induced haematological disorders. Though, the treatment with NJE before and after the electron beam irradiation in rats provided significant protection, further detailed studies in this direction are warranted, in order to use this herbal product in humans against radiation induced injuries. This study predicted that the treatment with NJE both before and after the irradiation is necessary to provide significant protection against the irradiation induced haematological damage. The CFU-S assay and the splenic index form valuable tools for demonstrating the repopulation of the entire haematopoietic organ in irradiated individuals. This assay is based on the fact that the total-body irradiation leads to the formation of colonies of proliferating cells in the spleens. These colonies appear as gross nodules in the spleens, which can readily be counted [27]. In the present study, no colonies were observed in the control rats. But, on irradiation, the appearance of gross nodules in the spleens was found to be highly significant. This was significantly increased by the NJE treatment in irradiated rats. The nodules which were observed in the spleens of the irradiated rats 15 days after the NJE treatment were discrete, round or oval, gray in colour, and they were embedded in the red mass of the spleen, as has been reported in other studies [27]. In the present study, the rats which were treated with NJE in rats and were exposed to EBR showed a significant increase in the splenic index. When the spleens are damaged or removed, such individuals are more susceptible to infections, and hence the splenic index is a suitable parameter for monitoring the immune system function. The significant increase in the splenic index, which resulted from EBR, indicated splenomegaly in the adult male Albino rats, which was concurrent with the results of a previous research which was done on other models [38]. Further studies need to be conducted to investigate the mechanism of action, metabolism, long term safety and the side effects of NJE, as well as its interactions with other natural products which are consumed as dietary components.
CONCLUSION
The radioprotective efficacy of NJE has been confirmed. The results of this study also indicated that the extract of N. Jatamansi could possibly serve as an acceptable blood booster. Extensive in vivo studies are warranted to use NJE clinically for radioprotection.
Note: P value, NS=Non significant, * = 0.05, ** = 0.01, *** = 0.001 between control and experimental groups.
[1]. Gowda KM Damodara, Shetty Latika, Krishna A P, Kumari N Suchetha, Sanjeev Ganesh, Naveen P, Protective Effect of Nardostachys Jatamansi Root Extract against Radiation Induced Damage on Liver and Kidney Functions in Albino Wistar RatsJournal of Pharmacy Research 2011 4(8):2462-65. [Google Scholar]
[2]. Maurya Dharmendra K, Devasagayam Thomas P, Nair Cherupally Krishnan K, Some novel approaches for radioprotection and the beneficial effect of natural productsIndian Journal of Experimental Biology 2006 44:93-114. [Google Scholar]
[3]. Jackson SM, The clinical application of electron beam therapy with energies up to 10MeVBr. J. Radiol. 1970 43:431-40. [Google Scholar]
[4]. Devi Uma P, Ganasoundari A, Vrinda B, Srinivasan KK, Unnikrishnan MK, Radiation Protection by the Ocimum Flavonoids Orientin and Vicenin: Mechanisms of ActionRadiat. Res 2000 154:455-60. [Google Scholar]
[5]. Gupta NK, Hypolipedimic action of garlic unsaturated oils in irradiated miceNat. Acad. Sci. Lett 1998 11:401-03. [Google Scholar]
[6]. Pande S, Kumar M, Kumar A, Investigation of Radio protective efficacy of root extract of Panax GinsengPhytother. Res. 1998 12:13-17. [Google Scholar]
[7]. Pande S, Kumar M, Kumar A, Investigation of Radio protective efficacy of Aloe Vera leaf extractPhar. Biol. 1998 36.1-6. [Google Scholar]
[8]. Goel HC, Prasad J, Sharma AK, Protective effects of Podophyllum against radiation damageAdv. Radiat. Biol. Peace. 1999 2:27-33. [Google Scholar]
[9]. Samarth RM, Kumar A, Radioprotection of swiss albino mice by plant extract of MenthapiperitaJ. Radiat Res. 2003 44:101-09. [Google Scholar]
[10]. Kumar A, Verma S, Kumar S, Radiomodifying effects of spirulina1st Int.Cong.Trad. Med and Mat. Med. 2000 4:30-34. [Google Scholar]
[11]. Saini MR, Kumar S, Devi Uma P, Late effects of whole body irradiation on the Peripheral blood of mice and its modification by liv-52Radiobiol. Radiother 1985 26:487-90. [Google Scholar]
[12]. Baliga MS, Jagetia GC, Venkatesh P, Reddy R, Ulloor JN, Radio protective effect of abana, a polyherbal drug following total body irradiationThe British Journal of Radiology 2004 77:1027-35. [Google Scholar]
[13]. Nunia V, Sancheti G, Goyal PK, Protection of Swiss albino mice against whole-body gamma irradiation by diltiazemThe British Journal of Radiology 2007 80:77-84. [Google Scholar]
[14]. Hosseinimehr SJ, Nemati A, Radio protective effects of hesperidin against gamma irradiation in mouse bone marrow cellsThe British Journal of Radiology 2006 79:415-18. [Google Scholar]
[15]. Saini MR, Kumar S, Jagetia GC, Saini N, Effect of Liv-52 against radiation sickness and mortalityInd Pract. 1984 37:1133-38. [Google Scholar]
[16]. Jagetia GC, Baliga MS, Maligi KJ, Sethukumar Kamath M, The evaluation of the radio protective effect of Triphala (an ayurvedic rejuvenating drug) in the mice exposed to gamma radiationPhytomedicine 2002 9:99-108. [Google Scholar]
[17]. Naik GH, Priyadarshini KI, Naik DB, Gangabhagirathi R, Mohan H, Studies on the aqueous extract of Terminalia chebulaas a potent antioxidant and a probable radioprotectorPhytomedicine 2004 11(6):530-38. [Google Scholar]
[18]. Nishteswar K, In Text book of Dravyaguna 2007 New DelhiChaukhamba publishing house:16-18. [Google Scholar]
[19]. Sharma PV, Dravyaguna Vignana 2001 21New DelhiChaukhamba publishing house:24 [Google Scholar]
[20]. Jagetia Ganesh Chandra, Baliga Manjeshwar Shrinath, The Evaluation of Nitric Oxide Scavenging Activity of Certain Indian Medicinal Plants In Vitro: A Preliminary StudyJournal of Medicinal Food 2004 7(3):343-48. [Google Scholar]
[21]. Singh Inder, Sharma Abhilasha, Jindal Archana, Soyal Dhanraj, Goyal PK, Fruit Extract of Emblica officinalis (Amla) Protects Radiation Induced Biochemical Lesions In The Brain of Swiss Albino MiceAnnals of Neurosciences 2006 13(3):65-71. [Google Scholar]
[22]. Pandey S, Sharma M, Chaturvedi P, Tripathi YB, Protective effect of Rubiacordifoliaon lipid peroxide formation in isolated rat liver homogenateInd. J. Exp. Biol. 1994 32:180-83. [Google Scholar]
[23]. Jagetia GC, Baliga MS, Venkatesh P, Ulloor JN, Influence of ginger rhizome (Zingiber officinale Rosc) on survival, glutathione and lipid peroxidation in mice after whole-body exposure to gamma radiationRadiat Res. 2003 160:5845-92. [Google Scholar]
[24]. Devi Uma P, Ganasoundari A, Radioprotective effect of leaf extract of Indian medicinal plant Ocimum sanctumInd J Exp Biol. 1995 33:205-09. [Google Scholar]
[25]. Phadke SA, Kulkarni SD, Screening of in vitro antibacterial activity of Terminalia chebula, Eclipta alba and Ocimum sanctumInd J Med Sci. 1989 43:113-17. [Google Scholar]
[26]. Arora RB, Nardostachys Jatamansi-a chemical, Pharmacological and clincial appraisalIndian Council of Medical Research. 1965 51:19-26. [Google Scholar]
[27]. Till JE, McCulloch EA, A direct measurement of the radiation sensitivity of normal mouse bone marrow cellsRadiation Research 1963 14:213-22. [Google Scholar]
[28]. Farong Yua, Fahong Yub, McGuireb PM, Lic R, Wangc R, Effects of Hydrocotylesibthorpioides extract on transplanted tumors and immune function in micePhytomedicine 2007 14:166-71. [Google Scholar]
[29]. Jagetia GC, Aruna R, The herbal preparation abana protects against radiation-induced micronuclei in the mouse bone marrowMutat Res. 1997 393:157-63. [Google Scholar]
[30]. Murray RK, Granner RK, Mayes PA, Rodwell VW, Red and White Blood CellsIn: Harpers Biochemistry 2000 USAMcGraw-Hill:780-86. [Google Scholar]
[31]. Saini MR, Kumar S, Jagetia GC, Saini N, Effect of Liv.52 against radiation sickness and mortalityIndPract. 1984 37:1133-38. [Google Scholar]
[32]. Jagetia GC, Ganapathi NG, Inhibition of clastogenic effect of radiation by Liv.52 in the bone marrow of miceMutat Res. 1989 224:507-10. [Google Scholar]
[33]. Jagetia GC, Ganapathi NG, Treatment of mice with a herbal preparation Liv.52.reduces the frequency of radiation induced chromosome damage in bone marrowMutat Res. 1991 253:123-26. [Google Scholar]
[34]. Kumar PV, Kuttan R, Kuttan G, Radioprotective effects of RasayanasInd J Exptl Biol. 1996 34:848-50. [Google Scholar]
[35]. Jagetia GC, Baliga MS, Cystone An ayurvedic herbal drug imparts protection to the mice against lethal effects of gamma-radiation: a preliminary studyNahrung. 2002 46(5):332-36. [Google Scholar]
[36]. Jagetia GC, Baliga MS, Treatment of mice with a herbal preparation mentat protects against the radiation-induced mortalityPhytootherapy Res. 2002 17:876-81. [Google Scholar]
[37]. Jagetia GC, Aruna R, The herbal preparation abana protects against radiation-induced micronuclei in the mouse bone marrowMutat Res. 1997 393:157-63. [Google Scholar]
[38]. Mendez Lopez M, Mendez M, Sanchez Patan F, Casado I, Aller MA, Lopez L, Corcuera MT, Alonso MJ, Nava MP, Arias J, Arias JL, Partial portal vein ligation plus thioacetamide: A method to obtain a new model of cirrhosis and chronic portal hypertension in the ratJ. Gastrointest. Surg. 2007 11(2):187-94. [Google Scholar]