MATERIAL AND METHODS
This study was conducted in the Department of Microbiology, J. N. Medical College Hospital, Aligarh, India for a period of one year and six months (2007-2008). It comprised of the samples that were received in the department from different wards, outpatient departments, Intensive Care Units (ICUs), nurseries and emergency wards. Various clinical specimens such as blood, pus, cerebrospinal fluid, urine, body fluids which included pleural, peritoneal and bile fluids, respiratory tract specimens like tracheal aspirates, bronchial aspirates, bronchoalveolar lavage fluid and ear and nasal swabs, catheter tips and drain tips were received in leak proof sterile containers. These samples were processed and identified by using the standard bacteriological tests [6]. The isolates which were identified as Citrobacter spp. from the specimens were further processed. A total of 105 randomly selected non repetitive bacterial isolates were included as the cases, whereas 20 isolates which were obtained from the stool samples from healthy subjects served as the controls for the study. The virulence markers which were studied were:
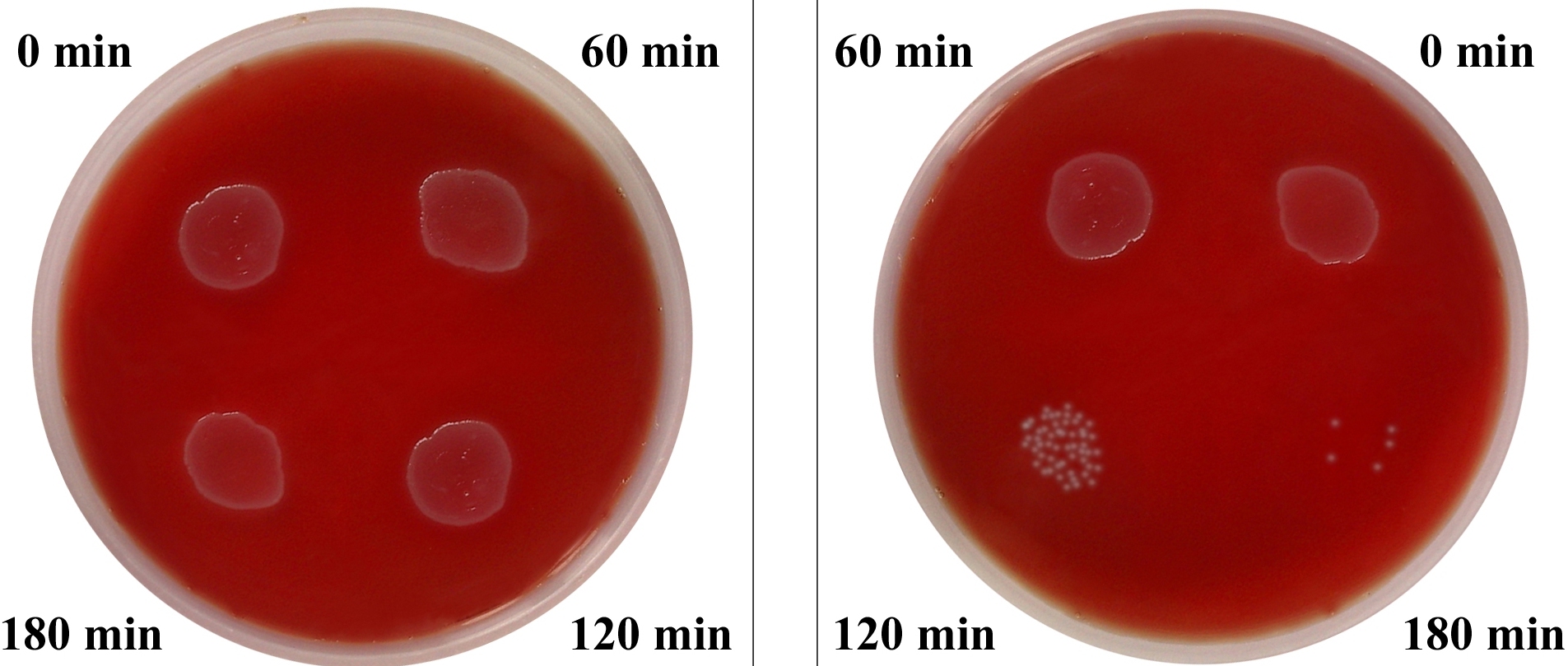
1. Serum bactericidal assay [4]: Overnight cultures of Citrobacter were harvested and cell suspensions were prepared in Hank's Balanced Salt Solution (HBSS) which contained 2.5x104 cfu/ml. To each well of a microtitration plate, 0.05ml of 100% serum was added. The control well contained 0.05ml of HBSS instead of serum. The samples (10μl) were withdrawn after incubation for 60, 120 and 180 minutes at 370C and spread on the blood agar plates which were incubated for 18hours at 370C and the viable counts were determined. The susceptibility of the bacteria and the serum bactericidal activity were expressed as the percentage of the bacteria which survived after 180 minutes with respect to the original count of the bacteria, which was determined at 0 minutes in the controls. According to Benge [7], the strains were termed as serum sensitive if the viable count dropped to 1% of the initial value and as resistant if more than 90% of the organisms survived after 180 minutes.
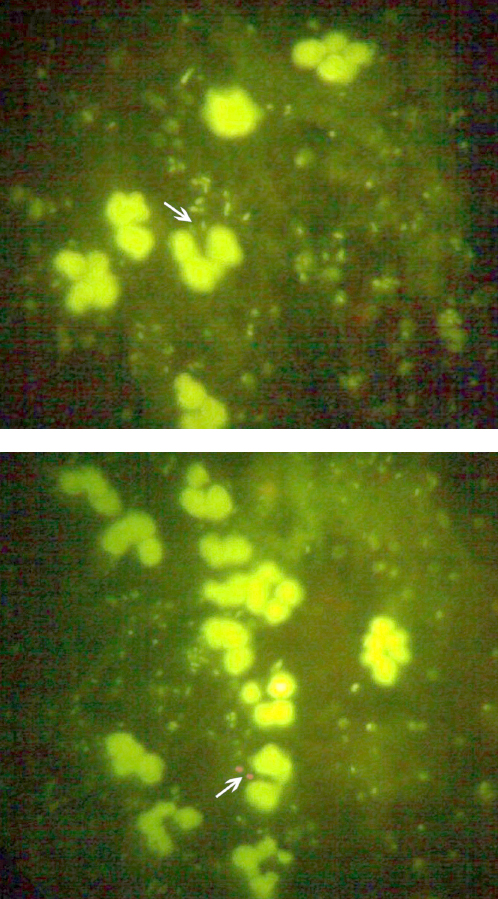
2. Intracellular killing of the Polymorphonuclear leucocytes [5]: The intracellular killing of bacteria by the human PMNLs was determined microscopically by making a slight modification in acridine orange staining method. Fresh unheparinised human blood (0.2ml) was placed on a sterile coverslip in a humidified plastic chamber and it was allowed to clot for 60 min at 37°C. The coagulated blood was removed by rinsing the coverslip gently in warm (37°C) HBSS. A PMNL monolayer was thus formed on the coverslip. The bacteria which were washed once in HBSS were pre-opsonized in 100% serum at 37° C (in a concentration of 3x107) for 15 min and 100 μl of this suspension was placed onto a PMNL monolayer. After incubation for 60 min, the coverslips were removed, air dried, stained with acridine orange (1mg/ml) for 1 min, rinsed in two changes of citrate phosphate buffer for 2 min each (pH-3.8) and mounted in phosphate buffered saline. In this assay, the intracellular bacteria fluoresced green when they were viable and red when they were non viable. The viability of the organisms in a total of 100 PMNLs was estimated [5]. The percentage killed was the percentage of the intracellular organisms that were non viable (red).

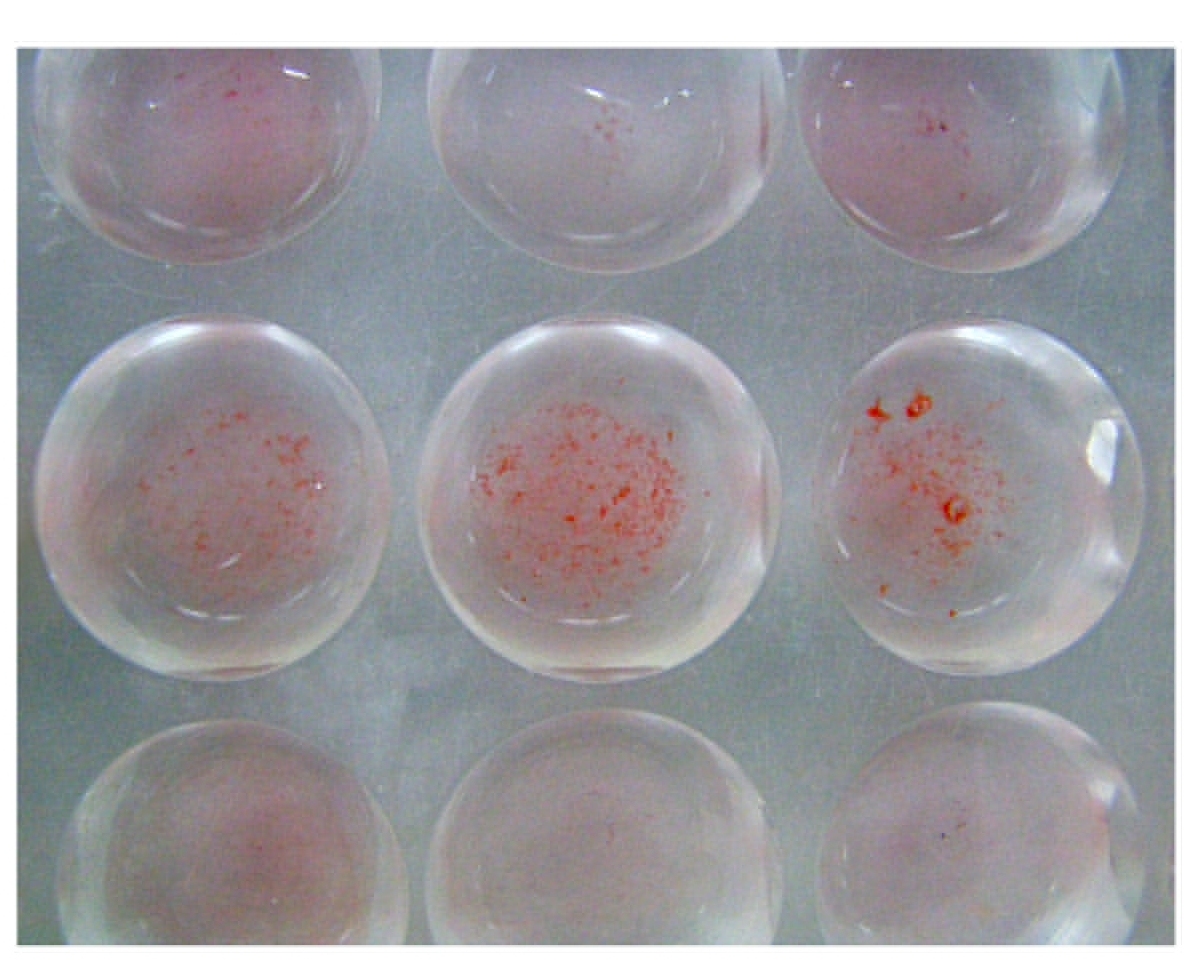
3. Cell Surface Hydrophobicity [4,7]: The cell surface hydrophobicity was tested by the 'Salt Aggregation Test' (SAT). The bacteria were tested for their hydrophobic properties by using different molar concentrations of ammonium sulphate. Those which aggregated with the salt particles and formed clumps were considered as hydrophobic. A strain of Escherichia coli which was haemolytic, mannose resistant, haemagglutination positive and consistently positive for the cell surface hydrophobicity was used as the positive control. A non pathogenic strain of Citrobacter which was obtained from the stool samples and was consistently negative for the cell surface hydrophobicity was used as the negative control. The isolates of Citrobacter were inoculated into 1ml of Phosphate Buffered Saline (PBS) which had a pH of 6.8 and the turbidity was matched with McFarland's tubes 6 and 7 to get a colony count of 5x109 colonies per ml. Different molar concentrations of ammonium sulphate, namely, 1M, 1.4M and 2M were prepared. Forty microlitres (40μl) of 0.2M PBS (pH-6.8) were taken in the first column of a cavity slide. Forty microlitres (40μl) of the 1M, 1.4M and 2M concentrations of ammonium sulphate were taken in each well of the other columns of this slide. Forty microlitres (40μl) of Citrobacter suspension was added to each of these wells. The clumps which were formed in different molar concentrations of ammonium sulphate were observed microscopically under the 10X and the 40X magnifications. The clumping was scored as positive on a one to four scale (1+ to 4+). The strains were considered as hydrophobic if they aggregated in concentrations of =1.4M. That is why, a lower and a higher concentration were selected. A tinge of Safranine was added to make the agglutinates visible to the naked eye. This was devised as an indigenous novel method for highlighting the colourless agglutinates.
RESULTS
The virulence markers were studied in 105 isolates from clinical samples and in 20 faecal isolates of Citrobacter from healthy subjects who formed the controls. The Salt Aggregation Test which was used to detect the cell surface hydrophobicity was positive in 17.1% (18) cases, while none of the control strains was positive. Eighty seven (82.9%) isolates from among the clinical cases were positive, whereas all the controls were negative for SAT. There was a significant (p<0.05) difference between the cases and the controls with respect to the cell surface hydrophobicity. Among the 18 isolates which gave a positive SAT with different concentrations of ammonium sulphate, 18(100%) gave a positive test with the concentrations of 2.0M and 1.4M, while 7(38.9%) were positive at the concentration of 1.0M. Since all the positive strains had a SAT value of <1.4M, all were considered as hydrophobic [Table/Fig-1].
Cell Surface Hydrophobicity: Salt Aggregation Test
Among the 105 Citrobacter isolates, 91(86.6%) were resistant to 100% serum, while 14(13.4%) were sensitive after 180 minutes of incubation [Table/Fig-2]. Out of the 20 faecal controls, 19(95%) were sensitive. The serum resistance among the cases was found to be statistically more significant as compared to that among the controls (p<0.001).Out of the 105 clinical isolates, the intracellular killing in the PMNLs in the range of 0-10% and 11-20% was found in 38.1% and 35.3% isolates respectively [Table/Fig-3]. Hence, in 73% strains, the intracellular killing in PMNLs was only in the range of 0-20%. Among the 20 control isolates, in 45% and 35% strains, the intracellular killing in PMNLs was in the range of 71-80% and 81-90% respectively. Overall, 80% of the control strains showed the intracellular PMNL killing to be in range of 71-90%. The mean killing of the isolates from the cases was 17±15 (SD) percent and it was 75±8.6 (SD) percent in the controls, which was significantly higher (p<0.001) [Table/Fig-4].
Serum Resistance Test Serum Resistant (Left) , Serum Sensitive (Right)
Intracellular killing in polymorphonuclear leucocytes. Viable bacteria flourose green (above) Non-viable bacteria flourose red (below)
Susceptibility of Citrobacter Isolates to Intracellular killing of PMNL: Comparision Of Cases with Controls
| Range of Citrobacter spp killed (%) | Isolates from |
|---|
| Cases (n = 105) | Controls (n = 20) |
|---|
| 0-10 | 40(38.1) | 0(0) |
| 11-20 | 37(35.3) | 0(0) |
| 21-30 | 17(16.2) | 0(0) |
| 31-40 | 2(1.9) | 0(0) |
| 41-50 | 3(2.9) | 0(0) |
| 51-60 | 2(1.9) | 1(5) |
| 61-70 | 2(1.9) | 2(10) |
| 71-80 | 1(0.95) | 9(45) |
| 81-90 | 1(0.95) | 7(35) |
| 91-100 | 0(0) | 1(5) |
| Mean (SD) | 17±15.7(SD) | 75±8.6(SD) |
The serum resistance and the cell surface hydrophobicity were not observed together. The presence of all three virulence markers was observed in 16 cases. Among the controls, only 1 strain was serum resistant. In the rest of the control strains, no virulence markers were present. The results of the presence of virulence markers in the cases and the control strains are shown in [Table/Fig-5].
Analysis of Presence of Virulence Markers in Citrobacter species isolated from Cases and Controls CSH : Cell Surface Hydrophobicity SR : Serum Resistance Intracellular killing of Polymorphonuclear leucocytes
| Groups | Virulence Markers | Cases (n = 105) | Control (n=20) |
|---|
| One Marker | | |
| 1. | Intracellular Killing | 12 | 0 |
| 2. | Csh | 0 | 0 |
| 3. | Sr | 2 | 1 |
| Total | 14 | 1 |
| Two Markers | | |
| 1. | Intracellular Killing+ Csh | 2 | 0 |
| 2. | Intracellular Killing + Sr | 73 | 0 |
| 3. | Sr + Csh | 0 | 0 |
| Total | 75 | 0 |
| Three Markers | | |
| 1. | Intracellular Killing + Csh + Sr | 16 | 0 |
DISCUSSION
In the present study, the presence of virulence markers in 105 isolates from various clinical samples and in 20 faecal samples which were taken as the controls, was evaluated. A recent study which pertained to the virulence markers in Citrobacter freundii was conducted by Li Bai et al., [8] in 2012, who found that the aggregative adherence and cytotoxicity played an important role in diarrhoea which was caused by the same organism. Taylor [9] reviewed that the bacteria are killed by normal human serum through the lytic activity of the alternate complement system. It has been suggested that the resistance to this bactericidal activity may be a significant virulence factor in Escherichia coli. The rough phenotypes are generally more sensitive to the serum killing than the smooth strains. Such a type of study on the serum resistance does not exist with respect to the Citrobacter species in the literature. We found a significantly high serum resistance in the cases as compared to that in the controls (p<0.001). Rizvi and Kumar [10] found a significantly high serum resistance in the urinary isolates of Escherichia coli as compared to that in the faecal isolates. On the contrary, Raksha et al., [11] did not find any significant difference between the cases and the controls for the serum resistance, though the Escherichia coli isolates from urine were more serum resistant as compared to the faecal controls. Olling et al., [12] found that the isolates of Escherichia coli from the patients of asymptomatic bacteriuria were more serum resistant than those from the patients of urinary tract infections (UTIs). Siegfried et al., [4] studied the serum resistance in the Escherichia coli isolates from children with respect to the type of UTI and found no significant difference in the serum resistance between the pyelonephritogenic strains of Escherichia coli and the strains which were isolated from the lower UTIs (58% Vs 68%).
Hydrophobicity has been considered a novel virulence mechanism of Escherichia coli. The role of the cell surface hydrophobicity in mediating bacterial adherence to the mammalian cells was conceived by Mudd and Mudd [13]. But the role of the cell surface hydrophobicity as a virulence marker in Citrobacter species, has not been documented in the literature. The difference in the cell surface hydrophobicity among the cases and the controls was found to be statistically significant (p<0.05). Siegfried et al., [4] found a very high percentage (76.8%) of hydrophobic Escherichia coli among the cases of UTI in their study. On the contrary, Raksha et al., [11] characterized uropathogenic Escherichia coli and found no significant difference in the CSH between the isolates from among the cases with UTI and the control faecal samples, though more isolates from the cases were hydrophobic.
An important virulence marker which was studied in our isolates was the intracellular killing of bacteria in the polymorphonuclear leucocytes. Originally, this virulence marker had been studied in Escherichia coli with reference to the production and action of alpha haemolysin. Bhakdi et al., [14] studied the effect of alpha haemolysin on the capacity of the polymorphonuclear leucocytes to mount a bactericidal phagocytic response. They reported that alpha haemolysin formed pores in the plasma membranes of the bacteria and led to their increased phagocytosis and intracellular killing. Seigfried et al., [5] also studied the intracellular killing in the polymorphonuclear leucocytes among the alpha haemolytic and the non haemolytic Escherichia coli isolates. They reported a significant difference between both (p<0.01). The range of the intracellular killing in the alpha haemolytic Escherichia coli was from 51-92% and the mean was 79.5±8.6(SD), while that of the non haemolytic Escherichia coli was from 58-97% and the mean was 84.4±6.9(SD). To the best of our knowledge, this virulence marker has not been reported in Citrobacter species and the strains are non-haemolytic. Hence, we studied it in comparison to the faecal controls. This was concordant with our study, in which 73% cases demonstrated intracellular killing in PMNLs in the range of 0-20%, while in 80% of the control strains, it was in the range of 71-90%. The killing among the controls was significantly high as compared to that in the cases (p<0.001).
To conclude, the virulence markers like the serum resistance, the cell surface hydrophobicity and the killing in the polymorphonuclear leucocytes, which had been relevant in Escherichia coli, were found to exist in Citrobacter spp. also. The colonization of these micro-organisms is a common feature. Hence, this study was designed to delineate the pathogenic Citrobacter isolates from the non pathogenic commensals/colonizing strains and these three markers served as useful tools for achieving this purpose. Citrobacter strains which did not show the presence of any of these virulence markers which were tested may represent colonization or contamination. Further research for establishing the cause and effect relationship of these virulence markers in Citrobacter, with the clinical conditions of the patients, is warranted.