Background and Objectives: Nitric oxide (NO) is a ubiquitous intercellular messenger molecule with important cardiovascular, neurological, and immune functions. In addition, it has been postulated that the pharmacological inhibition of NO or its actions may be therapeutically valuable in the disease management. The levels of nitric oxide may provide clues about the severity and the state of the underlying disease process. It could be an inflammatory biomarker that may enable clinicians to direct the environmentally based prevention or treatment programmes and to establish whether NO plays a role in the pathogenesis of periodontitis or not. Hence, the aim of the present study was to evaluate the salivary and the serum levels of NO in generalized chronic and aggressive periodontitis.
The Study Design: Unstimulated whole saliva and serum samples were collected from a total of 60 subjects who were in the age group of 18-45 years, who participated in this study. They were divided into three equal groups with 20 subjects in each group; group A (healthy controls), group B (chronic periodontitis) and group C (aggressive periodontitis). The clinical parameters were assessed, based on the oral hygiene index simplified (OHI-S), the gingival index (GI), the probing pocket depth and the clinical attachment loss (CAL). A biochemical analysis was performed to evaluate and compare the salivary and the serum nitric oxide levels of the above groups.
Statistical Analysis and Results: The statistical comparisons were done under the Griess Reaction. There were statistically significant salivary and serum levels of NO in the groups of periodontitis (group B and C) as compared to those in the healthy controls (group A). A significant positive correlation was found between the values of the salivary and the serum NO levels in chronic and aggressive periodontitis.
Conclusion: Nitric oxide is a potent modulator of the inflammatory disease processes and under pathological conditions, NO has damaging effects. As there is a paucity in the studies which have compared chronic and aggressive periodontitis, this study paved an interest for combining the serum and the salivary analysis in comparing the levels of nitric oxide in chronic and aggressive periodontitis.
INTRODUCTION
Periodontal disease is a chronic bacterial infection which is characterized by persistent inflammation, connective tissue breakdown and alveolar bone destruction.Although bacteria are probably the initiating agents in periodontitis, the complexity of the associated microflora and the critical role of the hosts in determining the outcome of the bacterial challenge may cause difficulties in clarifying the immunopathogenic mechanisms which are involved in the disease process [1].
Today, the advances in the diagnostic research of oral and periodontal disease are moving towards methods, whereby the periodontal risk can be identified and quantified by objective measures such as biomarkers.
A biomarker or a biologic marker is a substance that is objectively measured and evaluated as an indicator of the normal biologic processes and pathogenic processes, or the pharmacologic responses to a therapeutic intervention. Because serum and saliva can be easily collected and as they contain the locally and systemically derived markers of periodontal disease, they may offer the basis for a patient-specific biomarker assessment for periodontitis and other systemic diseases [2].
Nitric oxide (NO) is an ubiquitous intercellular messenger molecule with important cardiovascular,neurological and immune functions.Nitric oxide, a free radical gas, is a noxious chemical in the atmosphere, but in small controlled concentrations in the body, it acts as a physiological and pathophysiological mediator and it plays an important role in the biological systems [3].
In mammalian cells, NO is produced by a group of isoenzymes which are collectively termed as the NO synthases (NOS). All forms of NOS catalyze the conversion of L-arginine to L-citrulline in an NADPH dependent manner, producing NO from the terminal N-guanidino group of L-arginine. The NOS exist as three distinct isoforms, namely endothelial NOS (eNOS), Neural NOS(nNOS) and inducible NOS (iNOS). The endothelial NOS and neural NOS are constitutive and they release small amounts of NO for a short period following the stimulation of their receptors. In contrast, iNOS is expressed in response to proinflammatory stimuli and it produces large amounts of NO for sustained time periods [4].
The assessment of the stable end products of NO, nitrite and nitrate (NOx), is commonly used as a measure of the NO production in biological fluids. Saliva, serum, the gingival crevicular fliuid (GCF) and other biological fluids help us in determining the concentrations and the levels of various molecules from the diseased sites. A rapid serum diffusion of NO could contribute to the production of increased aqueous NOx, thus implicating NO in the pathophysiology and the progression of diabetic retinopathy as well as periodontal disease [5].
Recently, there has been an appreciation on how saliva could reflect virtually the entire spectrum of the health and disease states. These states include the tissue levels of the natural substances and a large variety of molecules which are introduced into the body for therapeutic, dependency or recreational purposes; the emotional status; the hormonal status; the immunological status; neurological effects; and nutritional and metabolic influences. Thus, saliva may contain biomarkers which are specific for the unique physiological aspects of periodontitis, and the qualitative changes which occur in the composition of these biomarkers could have diagnostic and therapeutic significance [6].
In addition, it has been postulated that the pharmacological inhibition of NO or its actions may be therapeutically valuable in the disease management. The levels of nitric oxide may provide clues about the severity and the states of underlying disease processes. It could be an inflammatory biomarker that may enable the clinicians to direct environmentally based prevention or treatment programmes.
As studies which pertain to the Indian scenario are sparse and since there is a paucity of studies which are focussed on aggressive periodontitis and also, with respect to the comparison of both serum and saliva, an attempt was made in this regards. Hence, the present study was carried out to compare both the serum and the salivary levels of NO in chronic and aggressive periodontitis and to establish whether NO plays a role in the pathogenesis of periodontitis or not.
MATERIAL AND METHODS
This study was planned and conducted during the period from September 2009 to November 2010 in the Department of Periodontics, Rajah Muthiah Dental College and Hospital, Annamalai University, India, to compare the nitric oxide synthase in saliva and serum as an inflammatory marker in periodontitis.
A cross-sectional study design was undertaken, with a sample size of 60, which was divided into 3 groups of 20 subjects each:
GROUP A (healthy controls) consisted of 20 subjects who were age and sex matched normal healthy individuals.
GROUP B (chronic periodontitis) consisted of 20 subjects who had a generalized probing pocket depth of ≥ 5mm.
GROUP C (aggressive periodontitis) consisted of 20 subjects who were diagnosed to have aggressive periodontitis, based on the AAP classification [7].
The inclusion criteria which was followed were patients of both genders who were in the age group of 25-55 years, with a dentition of at least 20 functioning teeth. Those subjects were excluded, who had systemic diseases, who were undergoing a periodontal treatment or who were on any anti-oxidant for the past 6 months, past and present smokers and patients with any salivary gland disorders.
The methodology was reviewed and permission was granted for the study by the ethical committee of our institution. The consents of the subjects were taken prior to the collection of the samples.
The detailed periodontal statuses of the patients who were included in the study were recorded by using the following periodontal parameters:
OHI (S) Greene And Vermillion
The gingival index according to Loe and Silness
The probing pocket depth
The clinical attachment level [8]
The Collection of Saliva
After recording the periodontal status, 2ml of unstimulated whole salivary samples were collected from all the four groups.
Each subject was first asked to rinse the mouth with distilled water to remove the food debris and to then spit into a sterile universal plastic container.
The subjects were instructed not to spit forcibly, so as to avoid a blood contamination, if any, from inflamed gingival tissues or the ulcerated lesions.
Once the saliva 2ml was collected, the plastic containers were placed in an ice carrier box and they were transferred to the laboratory for a biochemical analysis.
The saliva samples were centrifuged at 3000 rpm for 15 minutes
The supernatants were taken for the biochemical analysis and they were stored at 20°C until use.
The Collection of Serum
The consents of the subjects were taken prior to the collection of the samples.
About 2ml of venous blood was drawn from the patients’ arms to estimate the serum nitric oxide levels.
The blood samples were centrifuged at 3000 rpm for about 10 min to collect the sera, followed by the biochemical estimation of nitric oxide.
The Biochemical Estimation of Nitric Oxide
The nitric oxide levels were estimated by assaying the nitrite levels, a stable end product of the nitric oxide metabolism.
For the standard test: 1 M sodium nitrate was prepared and a double dilution was carried out according to the standard test table.
These solutions were added to the Griess reagent which was prepared by using 1% sulfanilamide, 1% naphthylethylene diamine dihydrochloride and 2.5% phosphoric acid [9].
The Griess reagent is very unstable, as it reacts with the surface atmospheric nitrogen. Hence, it was freshly prepared before use.
0.5 ml of the prepared standard solutions of sodium nitrite were reacted with equal volumes of the Griess reagent in Eppendorf tubes and they were incubated at room temperature for 10 minutes to ensure that a complete reaction took place.
The reaction mixture was then transferred into plastic cuvettes for measurement on a spectrophotometer which was connected to a computer, so that digital readings could be taken.
By using these readings which were taken for the standard solutions, a graph of the absorbance versus the concentration was plotted, which constituted the standard curve.
In a similar manner, the samples of the 60 subjects were added to the Griess reagent and they were then transferred to the spectrophotometer and their optical densities (OD) were recorded [10].
The optical densities were then correlated in the standard curve and the corresponding concentrations of nitrite were observed.
STATISTICAL ANALYSIS
The values which were obtained were subjected to a statistical analysis. The data which was obtained was subjected to a statistical analysis by using
Mean and standard deviation for each measured parameter
ANOVA-analysis of variance to determine the difference in the Nitric oxide values in different groups
A post-hoc comparison test to compare as to which particular pairs of groups were statistically different.
The Statistical Package for the Social Sciences (SPSS for windows, version 10.0) was used for the analysis. A p value of less than 0.05 was used to establish the statistical significance. The statistical comparisons were done by using the analysis of variance (ANOVA), followed by the Student’s paired ‘t’ and ‘f’ tests.
RESULTS
The detailed data on the salivary levels of NO between the three groups have been listed in [Table/Fig-1]. The detailed data on the serum levels of NO between the three groups have been listed in [Table/Fig-2]. The comparative values for the correlation between the salivary and the serum biochemical parameters have been listed in [Table/Fig-3].
ANOVA test for Comparison between three groups for Salivary Nitric oxide
| Groups | N | Mean | SD | Minimum | Maximum | f-value | p-value |
|---|
| Group A | 20 | 5.69 | 0.93 | 4.34 | 8.16 | 264.021 | 0.001 |
| Group B | 20 | 16.53 | 1.51 | 13.54 | 20.34 |
| Group C | 20 | 16.39 | 2.38 | 9.56 | 21.19 |
| Total | 60 | 12.87 | 5.39 | 4.34 | 21.19 | | |
P<0.01 Significant
Post Hoc Test of Salivary NO
ANOVA test for Comparison between three groups for Serum nitric oxide
| Groups | N | Mean | SD | Minimum | Maximum | f-value | p-Value |
|---|
| Group A | 20 | 4.89 | 1.05 | 3.28 | 7.17 | 210.793 | 0.001 |
| Group B | 20 | 15.75 | 1.97 | 11.66 | 20.34 |
| Group C | 20 | 15.43 | 2.42 | 10.48 | 18.94 |
| Total | 60 | 12.02 | 5.42 | 3.28 | 20.34 | | |
P<0.01 Significant
Post Hoc Test of Serum NO
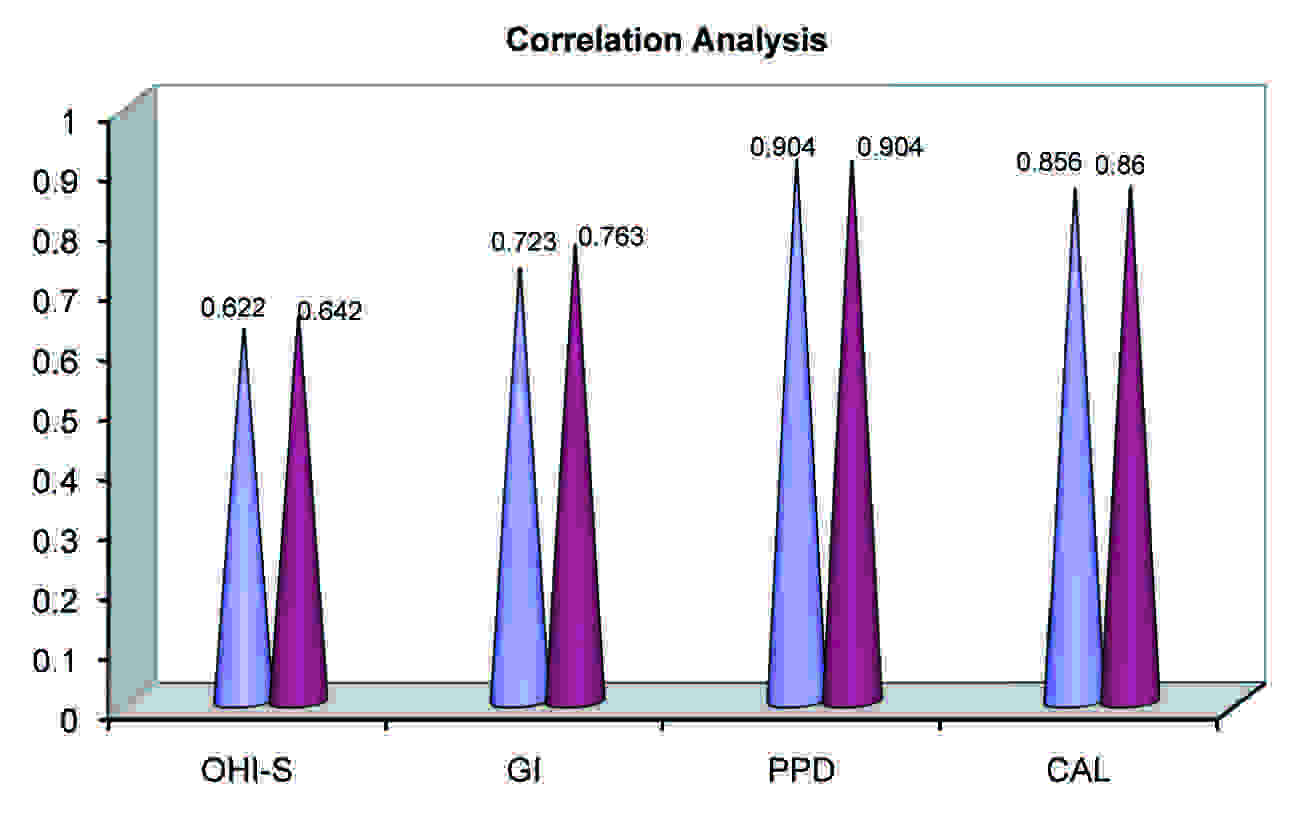
Pearson’s Correlation Coefficient Analysis
| OHI | GI | PPD | CAL |
|---|
| No Saliva | 0.622** | 0.723** | 0.904** | 0.856** |
| No Serum | 0.642** | 0.763** | 0.904** | 0.860** |
** Significant at 0.01 level.
The mean salivary nitric oxide score for Group A was 5.69 ± 0.93, for Group B, it was 16.53 ± 1.51 and for Group C, it was 16.39 ± 2.38. When the data was subjected to the ANOVA test, the f-value was found to be significant at a 0.001 level (f = 264.021: p < 0.001). The mean serum nitric oxide score for Group A was 4.89 ± 1.05, for Group B, it was 15.75 ± 1.97 and for Group C, it was 15.43 ± 2.42. When the data was subjected to the ANOVA test, the ‘f’ value was found to be significant at a 0.001 level (f = 210.793: p < 0.001).
When we compared the values of the above parameters, the results indicated that the serum and the salivary levels of NO were statistically significant in the study groups as compared to the healthy controls. There was no significant relationship between chronic and aggressive periodontitis. The results indicated that there was a positive significant relationship between the values of both the serum and the salivary NO levels with respect to their clinical parameters [Table/Fig-4].
DISCUSSION
Periodontitis is the general description which has been applied to the inflammatory responses of the gingival and the surrounding connective tissues to bacteria or plaque accumulations on the teeth. It is believed that while the primary aetiological agent is specific, which are the predominantly gram negative anaerobic or facultative bacteria within the subgingival biofilm, much the periodontal tissue destruction is caused by an inappropriate host response to those microorganisms and their products [11].
Nitric oxide (NO) is a gaseous free radical with a short biological half-life, which is generated enzymatically from L-arginine by a family of the NO synthase (NOS) isoforms. Nitric oxide (NO) is formed from the amino acid, L-arginine by a 2-step oxidation of L-arginine to L-citrulline. Nitric oxide is synthesized by a family of enzymes which are called nitric oxide synthases [12].
In the present study, we made an attempt to evaluate the roles of both the serum and the salivary biochemical parameters as inflammatory markers in periodontitis. Various studies have reported the significance of NO as an inflammatory marker, but the present study has correlated both the serum and the salivary elevated levels in chronic and aggressive periodontitis.
The expression of iNOS has been investigated in salivary gland-related diseases [13], in temporomandibular joint disorders and in oral cancer as well [14]. The importance of NO in the development of arthritis is increasingly being recognized. Several studies have suggested that the tissue injury in inflammation involves the induction of iNOS by certain cytokines or endotoxins, which leads to the production of large quantities of NO [15]. Hirose et al.,2001 have found that the NO production by macrophages and polymorphonuclear leukocytes via the iNOS pathway was enhanced in the periodontal lesions and that it had resulted in the progression of periodontitis [16].
The biochemical and the immunological markers which are present in saliva, serum or GCF can partially determine the extent of the periodontal disease and they may even predict its progression [17]. The collection of the salivary constituents is a simple, non-invasive procedure that can be performed by any individual. In addition, the obtained samples can be frozen and sent to laboratories for the analysis of markers such as NO [18]. In particular, the Griess reaction has potential as an auxilliary diagnostic tool, because it is a simple, highly specific and extremely sensitive method for measuring the micromolar concentrations of nitrite. Therefore, this method might be considered as an instrument for epidemiological research [19].
The source of salivary nitric oxide was studied by Sato H et al., [20]. Up to 25% of the plasma nitrate is actively taken up by the salivary glands and it is secreted with saliva, and the resulting salivary nitrate concentrations are at least 10 times higher than the concentrations in plasma. Many of the oral microorganisms express enzymes that can effectively reduce nitrate. The facultative anaerobic bacteria in the oral cavity reduce the salivary nitrate to nitrite and this nitrite enhances the gastric generation of NO in acidic conditions. Although nitrite is converted non-enzymatically to NO at a low pH, the rate of this conversion at the physiological pH (about 6.2-7.6) in the oral cavity is fairly low and, hence, the mechanism of the oral production of NO remains obscure.
The source of serum nitric oxide was studied by Menaka et al., [9]. The results of this study showed significantly increased concentrations of nitrite in the patients with periodontitis, as compared to those in the healthy control group. The significantly higher levels of NO in this study group had contributed to the development of the frequently found clinical symptoms of periodontitis. The gingival redness may be explained by the vasodilatory effect of NO, and the gingival swelling which was caused by the vascular permeability, which thusincrease the effect of NO. The increased tendency of the soft tissue to bleed on gentle probing may be due to the inhibitory effect of NO on platelet aggregation and the adhesion-inhibitory effect of NO. The increased alveolar bone resorption may be due to the stimulatory effect of NO on the activity of the osteoclasts.
The study which was done by Reher VG et al., [19] reported an increase in the salivary nitric oxide levels with an increase in the severity of periodontitis. The increased levels of nitric oxide in the periodontitis patients were attributed to the fact that there were increased levels of iNOS expressing cells during the inflammation of the periodontal tissue. Interestingly, a study report of Aurer A et al., [21], showed that salivary nitrite, a stable metabolite of NO, was decreased in the saliva of the periodontitis patients than in the healthy subjects. This may be due to the fact that NO is relatively unstable in the presence of oxygen and that it quickly autooxidizes to produce nitrogen oxides. Moreover, because of NO’s reactivity and short-life, directly measuring NO in the cells and tissues is very difficult.
The results from many studies have shown higher concentrations of L-arginine and L-citrulline in the inflamed gingival tissues, thus suggesting that the changes in the NO concentration occur in the patients with inflamed gingival tissues [22–24]. The inflamed periodontal tissues had demonstrated the iNOS expression at the baseline and the immunostaining had decreased after the periodontal treatment. Gullu C et al., [25] were the first ones to report the involvement of the arginine – NO pathway in chronic periodontitis. There were also statistically significantly increased levels of serum nitric oxide levels among the Groups B and C as compared to those in the healthy controls (Group A) which was consistent with the results of the study which was performed by Una M et al., [9, 24].
Since there is a paucity of studies in the literature which were focussed on the other forms of periodontitis like aggressive periodontitis, the purpose of the present case control study was to compare and correlate the nitric oxide levels in both the serum and saliva of generalized chronic periodontitis and generalized aggressive periodontitis patients with those of healthy individuals. Though many studies have revealed the alterations in the salivary NO concentrations, only few studies have reported on the serum NO levels. Hence, an attempt was made to compare the levels of salivary NO with respect to the serum NO concentrations. Pearson’s correlation coefficient (r) was used to evaluate the possible correlation between the NO concentrations and the clinical parameters. On the whole, we found that there was a significant positive relationship between the values of both the salivary and the serum NO concentrations.
CONCLUSION
Nitric oxide is a potent modulator of the inflammatory disease processes and under pathological conditions, NO has damaging effects. To summarize, as the studies which have highlighted on aggressive periodontitis are lacking and as for the biochemical tests, this study utilized easy chair side diagnostic tools i.e, saliva and serum, this study was indicated to focus on the relationship between the salivary and the serum levels of NO in generalized chronic and aggressive periodontitis patients. The results of our study indicated that there was a direct positive correlation between the salivary and the serum nitric oxide levels, which helped us to prove that the salivary non-invasive examination had a significant corelation with the serum analysis . Hence, the further studies should focus on larger sample sizes to confirm or refute the conclusions of this study and further research on the nitric oxide inhibitors may throw light on the drugs which can treat and manage periodontitis effectively.
P<0.01 Significant
Post Hoc Test of Salivary NO
P<0.01 Significant
Post Hoc Test of Serum NO
** Significant at 0.01 level.
[1]. Page RC, Kornman KS, The pathogenesis of human periodontitis: an introductionPeriodontol 2000 14(9):1997 [Google Scholar]
[2]. Biomarkers Definitions Working GroupBiomarkers and surrogate endpoints: preferred definitions and conceptual frameworkClin Pharmacol Ther 2001 69(3):89-95. [Google Scholar]
[3]. Lohinai Zslot M, Szabo Csaba, Role of nitric oxide in physiology and pathophysiology of periodontal disease.Review ArticleMed Sci Monit 1998 72:1089-95. [Google Scholar]
[4]. Michael T, Feron O, Nitric oxide synthesis: which, where, how and why?J. Clin. Invest 1997 100(9):2146-52. [Google Scholar]
[5]. Skaleric Uros, Gaspric Boris, Nancy Mc, Cartney-Francis. Potential Proinflammatory and antimicrobial Nitric oxide in gingival fluid of diabetic patients with periodontal diseaseInfection and Immunity 2006 74:7010-13. [Google Scholar]
[6]. David TW, Salivary diagnostics powered by nanotechnologies, proteonomics and genomicsJADA 2006 137:313-21. [Google Scholar]
[7]. Armitage GC, Development of a classification system for periodontal diseases and conditionsAnn Periodontol 1999 4:1-6. [Google Scholar]
[8]. Ramjford SP, Indices for prevalence and incidence of periodontal diseaseJ Periodontol 1959 30:51 [Google Scholar]
[9]. Menaka KB, Ramesh A, Thomas B, Kumari NS, Estimation of NO as an inflammatory marker in periodontitisJ Indian Soc Periodontal 2009 13:75-8. [Google Scholar]
[10]. Sharma KV, Saimbi CS, Mehrothra KK, Estimation of nitric oxide as a diagnostic marker of periodontal diseaseJISP 2003 6(3):137-143. [Google Scholar]
[11]. Lamster IB, Novak MJ, Host mediators in gingival crevicular fluid: implications for the pathogenesis of periodontal diseaseCrit Rev Oral Biol Med 1992 3:31-60. [Google Scholar]
[12]. Forstermann U, Schmidt HW, Pollock JS, Isoforms of NOS, characterization and purification from different cell typesBiochem Pharmocol 1991 42:1839-57. [Google Scholar]
[13]. Looms D, Tritsaris K, Pedersen AM, Nauntofte B, Dissing S, Nitric oxide signaling in salivary glandsJournal of Oral Pathol Med 2002 31(10):569-84. [Google Scholar]
[14]. Clancy RM, Amin AR, Abramson SB, The role of nitric oxide in inflammation and immunityArthritis Pheum 1998 41:1141-1151. [Google Scholar]
[15]. Stichtenoth DO, Frolich JC, Nitric oxide and inflammatory joint diseasesBr J Rheumatol 1998 37:246-257. [Google Scholar]
[16]. Hirose M, Ishihara K, Saito A, Nakagawa T, Yamada S, Okuda K, Expression of cytokines and inducible nitric oxide in inflamed gingival tissuesJournal Periodontal 2001 72:590-97. [Google Scholar]
[17]. Ugar-Cankal D, Ozmeric N, A multifaceted molecule, nitric oxide, in oral and periodontal diseaseClin Chim Acta 2006 366(1-2):90-100. [Google Scholar]
[18]. Sunitha M, Shanmugam S, Evaluation of salivary nitric oxide levels in oral mucosal diseases: a controlled clinical trialInd J Dent Res 2006 17(3):117-120. [Google Scholar]
[19]. Reher VG, Zenóbio EG, Costa FO, Reher P, Soares RV, Nitric oxide levels in saliva increase with severity of chronic periodontitisJ Oral Sci 2007 Dec 49(4):271-6. [Google Scholar]
[20]. Sato H, Takahashi M, Mutoh M, Shoji Y, Kamanaka Y, Naka M, Maruyama T, Sugimura T, Wakabayashi K, Suppressive effect of an inducible nitric oxide inhibitor, ONO-1714, on AOM-induced rat colon carcinogenesisNitric Oxide 2006 Mar 14(2):130-6. [Google Scholar]
[21]. Aurer A, Aleksic J, Ivic-Kardum M, Aurer J, Culo F, Nitric oxide synthesis is decreased in periodontitisJ Clin Periodontol 2001 Jun 28(6):565-8. [Google Scholar]
[22]. Matejka M, Partyka L, Ulm C, Solar P, Sinzinger H, Nitric oxide synthesis is increased in periodontal diseaseJ Periodontal Res 1998 Nov 33(8):517-8. [Google Scholar]
[23]. Kaufman E, Lamster IB, The diagnostic applications of saliva: a reviewCrit Rev Oral Biol Med 2002 13(2):197-212. [Google Scholar]
[24]. Una M, EI-Shinawi Mohammed EI-Saeed Elewa, Fagr EI-Shahaat, Role of nitric oxide and cotinine in periodontal diseaseJournal of Oral Sciences 2007 34:1483-87. [Google Scholar]
[25]. Güllü C, Ozmeric N, Tokman B, Elgün S, Balos K, Effectiveness of scaling and root planing versus modified Widman flap on nitric oxide synthase and arginase activity in patients with chronic periodontitisJ Periodontal Res 2005 Apr 40(2):168-75. [Google Scholar]