Peptic ulcer refers to an ulcer in the lower oesophagus, stomach or the intestinal mucosa [1]. The ulceration results when the aggressive factors (acid, pepsin, bile or H. pylori) overwhelm the defensive factors of the gastrointestinal mucosa (mucous and bicarbonate secretions, prostaglandins and nitric oxide) [2]. It affects 8-10% of the global population [3]. Although, the epidemiological studies show the high prevalence of the disease in the developing countries [1], twenty five million (approx.) Americans suffer from peptic ulcer diseases [4].
MATERIAL AND METHODS
A freshly prepared sample of Avipattikar churna (batch no. SAC-184) was obtained from Singhadurbar Vaidyakhana Vikas Samiti, Anamnagar, Kathmandu, Nepal. The reference drug, ranitidine (batch no. 67-68/503/01) was obtained from National Health Care P. Ltd., Chhatapipara, Birgunj.
Experiment site: This study was conducted in September 2011 at the pharmacology laboratory of the School of Health and Allied Sciences, Pokhara University, Nepal, after obtaining clearance (Ref. No. 101/2068-069) from the Research and Ethical committee of the university.
Animals: Sprague Dawley male rats (200-350g) were housed individually in polypropylene cages under standard husbandry conditions.They had been given the standard diet [12], protein-20%, carbohydrate-60%, fat-5% and fibre-5% and water ad. libitum.
Twenty four rats were divided into four groups, with six animals in each group. They were fasted with free access of water for a period of 24 hours, to ensure a complete gastric emptying and a steady state gastric secretion. The group I rats served as the ulcer controls and they received vehicle [2 % (v/v) gum acacia], group II served as the churna low dose (CLD) group and received the churna (500 mg/kg), group III served as the churna high dose (CHD) group and received the churna (750 mg/kg) and group IV served as the ranitidine reference control group and received ranitidine (25 mg/kg).
Gastric ulcer induction in the rats: Shay’s pyloric technique (1945) was used to induce gastric ulcers in the rats [13, 14]. The churna and ranitidine were suspended in the vehicle (2% (v/v) gum acacia) and they were administered via oral route with the help of feeding tube. The drugs were administered twice a day, with a dosing interval of 12 hours, for two days. After 1 hour of the last dose, pyloric ligations were performed in all the rats under general anaesthesia (ether). The pyloric ligated animals were housed separately in their groups. Food and water were withheld for a duration of 4 hours. Finally, all the rats were sacrificed by using an overdose of the ether anaesthetic. The stomach of each rat was removed separately after tying the lower oesophageal end. Subsequently, the stomach was incised along the greater curvature and it was rinsed with 2ml of distilled water. The collected gastric contents were centrifuged at 1500rpm for 10 minutes. The total volume of the supernatant liquid was measured and it was expressed in terms of ml/100g of the body weight of the rats. The pH was determined by using a digital pH meter. For the estimation of the total acidity, the diluted supernatant was titrated with 0.01N NaOH by using the Phenolphthalein reagent as an indicator [14].
After rinsing the stomach with normal saline (pH 7.0), it was stretched on a frog-board and with the help of a magnifying glass, the degree of ulceration was graded from zero to five. The graded number was expressed as the ulcer score [13–15]. The length of each ulcer in an individual stomach was measured with the help of a millimetre (mm) scale and it was summed up, which was denoted as the Gastric irritancy size (GIS). The total number of ulcers per stomach gave the Ulcer Number (UN). The Gastric Irritancy Index (GII) was then calculated by multiplying the GIS with the UN [16]. The difference in the average length of the ulcers in the control and the treated groups was also calculated. The difference which was divided by the average length of the ulcer in the control group and multiplied by hundred was expressed as the curative ratio [17].
Histopathological evaluation: The tissue specimens which were preserved in formalin were accessioned separately and processed through the steps of dehydration (with ethanol), clearing and embedding (with paraffin). Thus formed paraffin blocks were cut by using a rotary microtome. The sections were then fixed onto glass slides, stained with eosin and evaluated.
STATISTICAL ANALYSIS
SPSS (Statistical Package for the Social Sciences), version 17 was used to analyze the data. The results were presented as MEAN ± SEM. The data were processed for obtaining a ‘p’ value by one way ANOVA, followed by the Dunnett test. A ‘p’ value of less than 0.01 was considered as statistically significant.
RESULTS
[Table/Fig-1] shows the various parameters in the different groups. The difference in each group was compared individually with the results of the control group and the statistically significant (p<0.01) results were marked by (*). Similarly, the difference in each group was also compared individually with the results of the ranitidine treated group and the statistically significant (p<0.01) results were marked by the addition sign (+).
Various parameters in different groups with reference to ulcer control group and ranitidine treated group
| Groups\Parameters | Ulcer Control (mean± SEM) | Churna (500mg/kg) (mean± SEM) | Churna (750mg/kg) (mean± SEM) | Ranitidine (25mg/kg) (mean± SEM) |
|---|
| Volume of gastric content (ml/100g) | 4.6150±0.118+ | 1.338±0.177* | 1.675±0.270* | 1.980±0.518* |
| pH | 3.3017±0.145+ | 4.795±0.235* | 4.263±0.141* | 4.181±0.302* |
| Acidity | 153.216±1.976+ | 91.016±1.163* | 111.25±2.042 | 78.366±6.095* |
| ulcer score | 2.000±0.632+ | 0.750±0.483* | 0.750±0.316* | 0.750±0.795* |
| length of ulcer (mm) | 12.733±1.063+ | 0.628±0.563* | 0.986±0.548* | 0.5233±0.456* |
| No. of ulcer | 4.333±1.123 | 2.666±0.500 | 2.166±0.480 | 2.166±0.511 |
| GII | 60.800±5.381+ | 1.510±0.661* | 1.910±0.503* | 1.200±0.630* |
| Curative ratio | | 95.065 | 92.251 | 95.890 |
(Significant at* p<0.01 compared to control group, Significant at + p<0.01 compared to ranitidine treated group).
Evaluation of the parameters in comparison to those of the control group
The mean gastric contents of the ulcer control group was 4.615ml (maximum) and that of the CLD group was 1.338 (minimum). The mean volume of the gastric contents, acidity, the length of the ulcer and GII were significantly low in all the treated groups as compared individually with those in the control group. The decrease in the CLD group was significant as compared to that in the control group for all the parameters. The results among the churna treated groups (CLD and CHD) were not statistically significant at a p value of <0.01.
Evaluation of the parameters in comparison to those of the ranitidine treated group
When the results of the churna treated groups were compared separately with the results of the ranitidine treated group for various parameters, the differences which were observed were found to be statistically not significant.
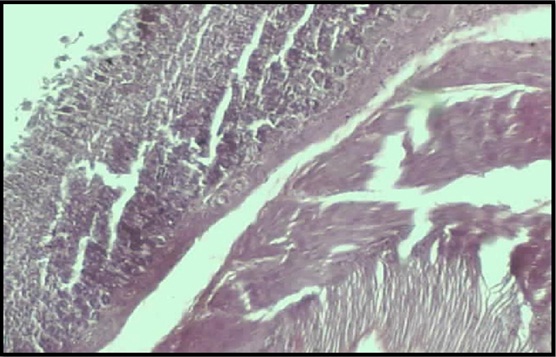
Histological examination of the gastric tissue: [Table/Fig-2] shows the normal architecture of the gastric tissues of the rats, with a distinct serosal layer, muscularis and the mucosal and the submucosal layers of the stomach. The photomicrograph of a stomach from the ulcer control group [Table/Fig-3] showed dead neutrophils with pus formation and fibrinopurlent exudates. The histological examination of the gastric tissues of the CLD and the CHD treated groups showed healing of the ulcers, with few inflammatory cells (eosinophills, lymphocytes and plasma cells) at the mucosal layer [Table/Fig-4 and 5] as compared to those of the ulcer control group. The architecture of the gastric tissue was less distorted (almost normal) in the ranitidine treated group [Table/Fig-6].
Photomicrograph of normal gastric tissue (10X) of rat showing normal mucosa, muscularis and serosal layer
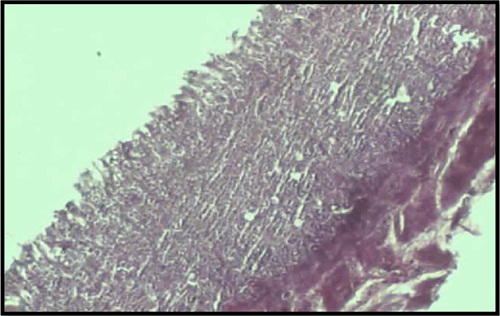
Photomicrograph of gastric tissue (10X) of rat from control group showing the damaged gastric architecture with dead neutrophils and pus formation
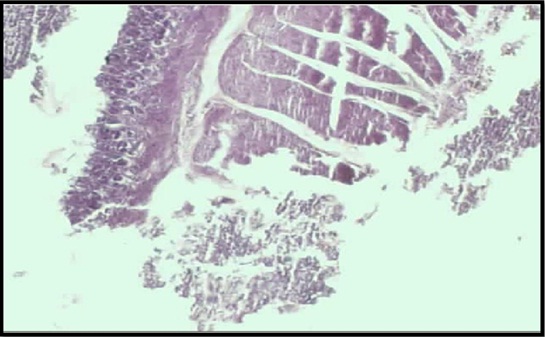
Photomicrograph of churna low dose treated gastric tissue showing few inflammatory cells presented only in the base of the mucosal layer (50X)
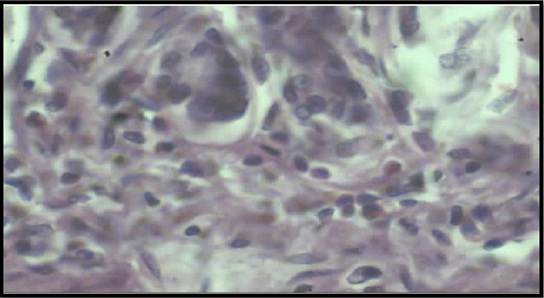
Photomicrograph of churna high dose treated gastric tissue showing distinct inflammatory cells present in the mucosal layer (50X)
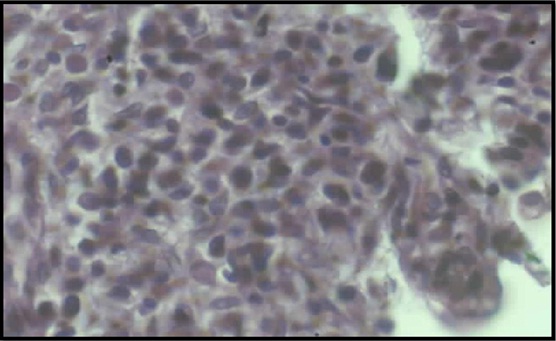
Photomicrograph of Ranitidine treated gastric tissue (10X)
DISCUSSION
There are various methods for the production of peptic ulcers in animals and pyloric ligation is one of the most commonly used methods [13, 14]. Pyloric ligation causes accumulation of the gastric juice and hindrance of the gastric blood circulation, which lead to damage of the upper gastrointestinal tract and the formation of ulcers [16, 18]. In our study, pyloric ligation significantly elevated the gastric acid secretion, acidity and the ulcer score and it decreased the pH of the gastric contents. In a similar type of study [19], the pyloric ligation was found to decrease the pH to 3.36 in the control rats. Similarly, it was reported to decrease the pH to 3.21 in another study [20]. In our study, the gastric pH decreased up to 3.30, which probably indicated that the ligation was effective in our study.
Ranitidine, a commonly used H2 blocker, inhibits the acid production by reversibly blocking the H2 receptors on the basolateral membrane of the parietal cells [1, 2]. The volume of the gastric juice in the ranitidine, CLD and the CHD treated groups were 1.9800 ± 0.518, 1.3383 ± 0.177 and 1.675 ± 0.270 ml respectively, which was significantly (p<0.01) less, as compared to that in the control group (4.6150 ± 0.118 ml). The results were comparable with those of a similar type of Indian study [19], where the mean volume of the gastric secretion in the ranitidine treated, Avipattikar churna extract (500mg/kg) treated and the control groups were 1.916, 2.466 and 4.25 ml respectively. The churna showed a good antisecretory activity in our study, which could have been due to the variations in the quality of the churna or the use of the raw drug in our study, unlike in the other study [19]. In Ayurveda, a raw drug (without extraction) is said to contain various components which work synergistically and give good effects with low side effects.
There were significant decreases in the acidity in the ranitidine and the CLD treated groups as compared to those in the ulcer control group. However, the decrease in the acidity in the CHD group was insignificant as compared to that in the control group. The pyloric ligation causes the accumulation of acid, which irritates the stomach wall and the stress which is caused by the ligation, further increases the irritation [18]. In all the treated groups, there were statistically significant decreases in the GII as compared to those in the ulcer control group. This indicated that the churna and ranitidine, both help in the prevention of GII. Furthermore, the differences in the GII among the treated groups were statistically insignificant, which signified that the churna was as effective as ranitidine in controlling the irritation. The differences in the curative ratios between the CLD (95.065), CHD (92.251) and the ranitidine (95.890) groups were also statistically not significant.
Ranitidine reduced the gastric acid secretion, it reduced the ulcer score and it increased the pH, thus showing an anti-ulcerogenic effect. The test drug, Avipattikar churna (especially at 500mg/kg dose) was found to be equally efficacious with the reference drug, ranitidine (25mg/kg). There were no significant differences among the CLD, CHD and the ranitidine treated groups with respect to the compared parameters. Although the CHD group received a higher dose (750mg/kg) than the CLD group (500mg/kg), the effect on the various parameters was almost the same (or some time less). At a 750mg/kg dose, the quantity which was administered became very high, to the extent that it could have led to indigestion and adversely affect the anti-secretory and the anti-ulcerogenic activities of the churna.
The histological evaluation of the gastric tissues of the rats from various groups also supported the results. The churna prevented the damage of the gastric tissues which could be caused by the excessive acid secretion and thus, it provided a less distorted gastric mucosal architecture which was comparable to that of the ranitidine treated rat gastric tissue. The inflammatory cells at the mucosal layer of the gastric tissue of the churna treated group were lesser than in the ulcer control group, which added evidence on the effective healing property of the Avipattikar churna. Similar types of histopathological results were also obtained in the study which was conducted by Aswatha RHN et al., [16].
LIMITATIONS
This study was limited to the ulcers which were caused by the pyloric ligation method only and so our results may not be applicable to the studies where the ulcers are produced by other methods. The quality of the churna which was used in this study was not assessed. Pharmacognostic tests e.g. the ash value, the saponification value, acid-insoluble ash, the water-soluble ash values, the moisture content, etc were not performed before the study due to time limitations.
CONCLUSION
The test drug, Avipattikar churna, showed anti-secretory and antiulcerogenic effects against the gastric ulcers which were induced by a pyloric ligation. The anti-secretory and the anti-ulcerogenic activities of the churna were similar to those of ranitidine. A good therapeutic effect was observed at a 500 mg/kg dose of the churna.
(Significant at* p<0.01 compared to control group, Significant at + p<0.01 compared to ranitidine treated group).