INTRODUCTION
Prenatal Ultrasound is a widely accepted tool for detecting fetal anomalies during pregnancy and, once detected, further investigations are instigated, including fetal chromosome analysis, maternal and fetal investigations for infections, microarray analysis, and fetal echocardiogram and magnetic resonance imaging, when indicated. Such battery of investigations projects complete knowledge of the cause, prognosis, and recurrence risk of the diagnosed clinical problem. Common practice is to perform an ultrasound evaluation of fetal anatomy transabdominally at 18 to 22 weeks’ gestation [1].
NT is the normal fluid filled subcutaneous space between the back of the fetal skin and the overlying skin. An increased NT was first described as a measure greater than 95th percentile for a given crown rump length. However, recent reports have highlighted that adverse outcomes are much more common with an NT that exceeds a set threshold of 3.5mm (i.e. R 3.5mm), a measurement that essentially represents 99th percentile or more throughout the gestational age window for first trimester screening [2]. NT detection by ultrasound has emerged as a powerful prenatal screening strategy to diagnose a myriad of syndromes, but per se cannot be applied as a stand-alone benchmark in detecting structural and karyotyping related anomalies.
Present review looks into the associations of NT with reported fetal abnormalities and explores the published literature about the potential of NT in surfacing certain fetal anomalies and incorporating this useful screening tool with adjunct ultrasonic parameters and serum markers for augmenting the diagnostic accuracy in first trimester.
STANDARD TECHNIQUE OF NUCHAL TRANSLUCECNY MEASUREMENT
NT measurement involves sonographically measuring the back of the fetal neck between 10 weeks 3 days and 13 weeks 6 days, and then comparing the measurement to NT nomograms at a given gestational age [3] [Table/Fig-1]. The recommended strategies for performing NT ultrasound are outlined in [Table/Fig-2].
Nuchal translucency as seen in the 13th week of gestation in a normal foetus
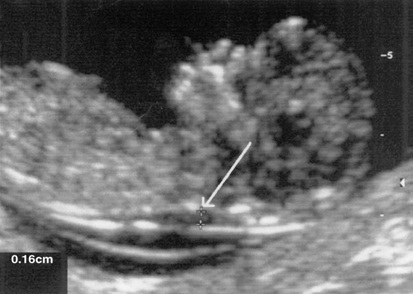
Sonographic criteria to maximize quality of nuchal translucency sonography
Nuchal translucency ultrasound should only be performed by sonog raphers or sonologists trained and experienced in the technique. Transabdominal or transvaginal approach should be performed, based on maternal body habitus, gestational age, and fetal position. Gestation should be limited to between 10 weeks 3 days and 13 weeks 6 days (approximate fetal crown–rump length, 36–80 mm). Fetus should be examined in a midsagittal plane. Fetal neck should be in a neutral position. Fetal image should occupy at least 75% of the viewable screen. Fetal movement should be awaited to distinguish between amnion 7. and overlying fetal skin. Calipers should be placed on the inner borders of the nuchal fold. Calipers should be placed perpendicular to the long axis of the fetal body. At least three nuchal translucency measurements should be obtained, with the mean value of those used in risk assessment and patient counseling. At least 20 minutes might need to be dedicated to the nuchal trans lucency measurement before abandoning the effort as failed.
|
Adapted from Malone FD and D’ Alton ME [3].
The largest prospective trial to date comparing 12 to 14 weeks’ with an 18 to 22 weeks’ anatomy examination did not find a detection rate advantage but the distinct ability to provide options for pregnancy management at an earlier date [4].
NUCHAL CORD AND ITS CLINICAL EFFECTS
‘Nuchal cord’ refers to umbilical cord encircling the fetal neck. Nuchal cord can have clinical implications on NT and nuchal fold thickness (NFT) measurements, in the first and second trimesters of the pregnancy, respectively [5]. Nuchal cord can cause indentation of the fetal skin, thus causing displacement of the fluid in the fetal neck leading to alteration of the NT measurement [6]. Other possible mechanisms that are listed to explain the increased NT measurements of nuchal cord fetuses are the alterations in the lymphatic drainage and transient heart failure due to umbilical cord compression. Taipale et al., [7] have shown that there is a learning curve in ultrasound detection of fetal anomalies in early pregnancies at 13 to 14 weeks. Although the detection rate was only 22% during the beginning of their study, it increased to 79% during the fourth year of the study. Bronshtein and Zimmer [8] have emphasized the importance of experience and training in early ultrasound, suggesting that a high detection rate is more likely after proper training.
IMPLICATIONS OF NUCHAL TRANSLUCENCY AND PROTOCOLS TO TACKLE
The association between increased NT and adverse pregnancy outcome in karyotypically normal fetuses has been established by several researchers [9]. Sairam et al., have reported that the presence of an increased NT thickness with normal karyotype should be recognized as an independent risk for fetal cardiac and extra-cardiac defects [10]. Following initial reports from Hyett et al., [11] several papers have established that the prevalence of congenital heart diseases increases with increasing NT thickness. Clur et al., [12] have shown from pooled data that this increases from 1.5% (in fetuses with NT b3.5 mm) to 3.4% (if NT is 3.5 to 4.4 mm), 7.5% (if NT is 4.5 to 5.4 mm), 15% (if NT is 5.5 to 6.4 mm), 19% (if NT is 6.5 to 8.4 mm) and 64% (if NT is >8.5 mm). In chromosomally normal fetuses with increased NT, the risk of miscarriage [Table/Fig-3], intrauterine death, diagnosis of major structural defects (including CHD) leading to termination of pregnancy, postnatal death or survival with defects also increases with increasing NT [13]. The probability of an overall adverse outcome in chromosomally normal fetuses with increased NT for the group with NT of 3.5 to 4.4 mm was 14.1%, 22.7% for those with NT of 4.5 to 5.4 mm, 30.7%, for those with NT of 5.5 to 6.4 mm, and 68.8%.for those with NT ≥6.5 mm [14]. More recently, Westin et al., have shown that when compared to the general population, NT ≥3 mm increased the likelihood of adverse outcome by six-fold, NT ≥3.5 mm by 15-fold and NT ≥4.5 mm by 30-fold [15]. A similar trend of increasing prevalence of cardiac defects with increasing NT thickness is reported by other groups; prevalence of cardiac defects was 14.9/1000 for NT 2.5—3.4 mm and 62.7/1000 for NT >3.5 mm [16]. Providing this significant information at the time of counseling the prospective parents is mandatory in the decision-making process at an early stage of pregnancy.
Risk of miscarriage in the setting of thickened NT
| Nuchal Translucency (mm) | Fetal Death a (%) | Live Birth Without Abnormality b (%) |
|---|
| 3.5—4.4 | 2.7 | 70 |
| 4.5—5.4 | 3.4 | 50 |
| 5.6—6.4 | 10.1 | 30 |
| >6.5 | 19.0 | 15 |
a Fetal death in the absence of chromosomal abnormalities.
b Residual rate excluding fetal death, major abnormalities, or chromosomal abnormalities.
In specialized centers, the management protocol of pregnancies with increased fetal NT encompasses invasive testing, followed by a detailed scan at 18—20 weeks to diagnose or rule out major (structural) abnormalities, with the option of fetal echocardiography, as and when required. Pregnancies could benefit from an early anomaly and early echocardiography well before the routine scheduled US scan at 18—20 weeks of gestation. In the presence of an important abnormality, 77% of parents chose to terminate the pregnancy [10], while at another center the termination rate was 66% after detection of structural defects [17]. In centers where expertise is available, fetal echocardiography is offered for cases with increased NT and normal karyotype, in the first trimester or soon afterwards. Several reports in the published literature have described the effective role of transvaginal US scans in fetuses less than 14 weeks gestation in early diagnosis of CHDs [18].
Prospective studies have demonstrated that 40% of major defects may be detected using the 4-chamber view alone, increasing to 70% by including views of the outflow tracts in routine screening. Mean NT measurements increase by approximately 17% each week from 10 to 14 weeks’ gestation [19]. Therefore, using a single millimeter cutoff to define an abnormal nuchal translucency is not justified. More appropriate options by using the 95th percentile for a particular gestational age or MoMs should be considered in equivocal cases.
Recent research using a combination of increased NT, tricuspid regurgitation and abnormal flow in the ‘a’ wave of the ductus venosus can be a substantial screening strategy to predict major cardiac defects at this early stage of gestation [20]. Postnatally, nuchal thickening is recognized as a webbed neck, and is typically associated with aneuploidy, cardiac defects and lymphatic abnormalities. Berdahl et al., explored the Iowa Birth Defects Registry to report the incidence of CHD in infants with and without a webbed neck [21]. They described a high prevalence of aneuploidy, genetic syndromes or evidence of lymphatic obstruction sequence in infants with a webbed neck.
NUCHAL TRANSLUCECNCY AND DOWN SYNDROME
A diverse range of protocols and strategies have been reported in the published literature which can be applied in the detection of DS using the NT and a variety of serum markers during different stages of the gestation. The most effective sonographic marker of trisomy 21 and other chromosomal defects is increased NT at 11—14 weeks [22]. Certain algorithms and formulae have been devised for stratifying individual patient risk for trisomy 21 by NT in combination with maternal age and with various maternal serum biochemical markers [13].
A. Nuchal transparency and first-trimester serum markers; Combined testing
The most popular first-trimester Down syndrome (DS) screening protocol includes three independent markers: maternal serum levels of PAPP-A, free or intact hCG, and measurement of fetal NT [23]. The timing plays a pivotal role which substantially affects the performance of both serum analytes and NT; the discrimination of free hCG improves with increasing gestational age and is greatest at 13 weeks, whereas PAPP-A performs optimally at 10 weeks and declines afterwards [24]. Consequently, when free hCG and PAPP-A are performed together, they complement each other and provide a consistently high sensitivity from 11 to 13 weeks. Several reports have employed varying definitions of an “enlarged” NT, but generally used size cut-offs range from 2 to 10 mm [25]. Stewart and Malone summarized the findings of 22 such early reports, including a total of 1875 high-risk pregnancies of which 30% were aneuploid, and found that an abnormal NT correctly identified fetal DS in 46% to 62% of cases [26]. The accuracy of NT screening in detecting DS ranges from 29% to 100%, at false-positive rates of 0.3% to 11.6% [27]. A significant number of the published studies do not indicate an accurate success rate at obtaining a NT measurement [28]. However, some studies have demonstrated a 100% success rate at obtaining a NT measurement [29] but none provide information on the adequacy of these images once obtained [3].
The first-trimester maternal serum screening has consistently revealed that pregnancies with fetal DS are associated with higher levels of total hCG and of the free hCG (with a median multiple of the median [MoM] of 1.83 in affected cases) and lower levels of pregnancy-associated plasma protein A (with a median MoM of 0.38 in affected cases) [30]. These first-trimester serum markers are described independent of NT, which would imply that a unified protocol using both serum markers and ultrasound can be applied with more accuracy than either alone. Detection rates for this combined approach from a series of four studies summarized that from a total of 85,412 patients screened in these studies, the overall sensitivity for detecting DS was 82% for a 5% false-positive rate [28] [31] [32] [33].
B. Nuchal translucency and first and second trimester serum markers; Integrated testing
This two-step testing involves a combination of NT and pregnancy-associated plasma protein A in the first trimester with serum AFP, hCG, unconjugated E3, and Inhibin-A in the second, with a single DS result being provided in the second trimester. The advantage of this approach stems from the fact that it can outline a very high sensitivity for DS as high as 94% for a 5% false-positive rate [34]. Controversy prevails about effectiveness and patients’ compliance of the integrated approach; those found to have a suspicion of DS during the first trimester have to wait till the second trimester screening is performed. This factor may lead to unnecessary anxiety for the patient and concerned family.
C. Nasal translucency and absence of nasal bone in first-trimester ultrasound
At 11—14 weeks of gestation the fetal nasal bone is cannot be visualized by ultrasound in about 60—70% of fetuses with trisomy 21 and in less than 3% of chromosomally normal fetuses [35].
The relationship of increased NT and absence of fetal nasal bone has been coined as an ultrasonic screening tool during the first-trimester but adequate visualization of the nasal bone needs expertise and correct technique. A study of 701 fetuses with increased NT evaluated the existence of fetal nasal bones and reported that a nose bone could not be visualized in 73% of DS fetuses (43 of 59) and in only 0.5% of unaffected fetuses (three of 603) [36]. This report was challenged by Hutchon et al. who described a series of five consecutive cases of DS with clearly visible nasal bones [37]. More evidence-based studies are needed to validate the importance of absent nasal bone as a screening marker for DS.
First trimester screening holds the promise of improved detection rates with lower false-positive rates. Serum, Urine and Ultrasound Screening Study (SURUSS) [38] and First and Second Trimester Evaluation of Risk for Fetal Aneuploidy (FASTER) [39] trials for the first time have allowed accurate comparison of currently available DS screening approaches in prospective studies of large populations.
NUCHAL TRANSLUCENCY AND CARDICAC ANOMALIES
The emerging effects and possible pathogenic mechanisms of enlarged NT include fetal heart failure secondary to a cardiac defect, anemia, infection, inappropriate expression of atrial natriuretic peptide; abnormal extracellular matrix; or abnormalities of lymphatic structure and drainage [40]. Enlarged NT leads to lymphatic obstruction which in its most severe form results in cystic hygroma. A cystic hygroma is a fluid-filled multi-septated cyst or cysts that arise from the back of the neck. When an enlarged NT or small cystic hygroma resolves before birth, the infant may be left with a webbed neck. Clark [41] and Lacro [42] reported a strong association between webbed neck and coarctation of the aorta in infants with Turner syndrome. In two reports including 205 Turner’s cases, infants were found to have webbed neck at birth, were 8 times more likely to have a congenital cardiac defect, especially aortic coarctation, than those without neck webbing. The reported association between NT-webbed neck and cardiac anomalies, both in fetuses with a variety of genetic syndromes and in euploid fetuses, points to the possibility of an established relationship. The lymphatic obstruction that leads to an enlarged jugular lymph sac could also cause lymph to accumulate in the thoracic duct. Due to its anatomical location, in the thoracic cavity, the enlarged thoracic duct might exert pressure on or displace the heart, causing obstruction of blood flow through the cardiac chambers, and leading to abnormal (inadequate) growth of certain cardiac structures. Cardiac anomalies believed to result from such abnormal intracardiac blood flow include aortic coarctation and hypoplastic left heart [43]. Currently, screening by fetal echocardiography is offered to the fetus following the observation of an NT of 3.5 mm or more [44]. The cost-effectiveness of offering fetal screening echocardiography at NT measurements of 2.5 to 3.4 mm has not been established.
Hyett and coworkers [45] evaluated the relationship between NT size and major cardiac defects in more than 29,000 euploid pregnancies, and described that the incidence of cardiac defects increased along with NT size; the prevalence was only 0.8 per 1000 fetuses with a normal NT, it increased to 28.9 per 1000 with an NT of 3.5 to 4.4 mm, 90.9 per 1000 with an NT of 4.5 to 5.4 mm, and 195.1 per 1000 with an NT 5.5 mm. Goetzl [46] has summarized results from seven studies that the incidence of cardiac abnormalities was positively related to NT; for NT of up to 3.5 mm the incidence was 6.0% (5.2%—6.8%), for 3.5-4.4 mm it was 3.2% (2.3%—4.1%), while for NT more than 4.5 mm the incidence of cardiac anomalies rose to 11.8% (9.8%—13.8%).
There is growing body of evidence that patients with increased fetal NT and normal karyotype are at higher risk of adverse outcome, cardiac or otherwise [10]. Cardiovascular anomalies are the most frequently encountered defects in chromosomally normal fetuses with increased NT. Based on such findings, early fetal echocardiography and anomaly scan should be considered in these fetuses. Patients also need to be informed, that in the presence of increased NT and a normal anomaly scan and fetal echo by 21—23 weeks, there is a 95% chance of a good outcome.
NUCHAL TRANSLUCENCY AND OTHER ANOMALIES
Enlarged NT has been reported with other structural anomalies, including diaphragmatic hernia, exompholos, body stalk anomaly, fetal akinesia syndrome, skeletal dysplasias, various multiple anomaly syndromes, and fetal loss [47, 48]. Keeping in view the published literature about the associations of enlarged NT, the euploid should also be evaluated by targeted second trimester ultrasound examination [49]. An increased NT has been associated with parvovirus infection [50]. If increased NT leads to signs of fetal hydrops at 20 to 22 weeks, parvovirus screening is recommended, in addition to evaluating the standard infections associated with fetal hydrops, such as toxoplasmosis and cytomegalovirus [46].
Associations of increased NT have also been described with cerebral hypoplasia [51], facial cleft [52], spine disorganization [53], hydrops and hepatomegaly [54], growth retardation [55], and skin edema [56].
THE LIMITATIONS OF NUCHAL TRANSLUCENCY-BASED SCREENING REPORTS
Ultrasound examination of the fetus is a subjective process that is highly dependent on operator skills and the quality of the sonographic equipment. These limitations militate against the deployment of ultrasound as a screening tool in the manner in which maternal serum biochemistry has been used [57].
CONCLUSION
Evaluation of the nuchal translucency should be considered during the first trimester ultrasound and a detailed anatomic evaluation should be offered whenever feasible. Increased NT is associated with a spectrum of fetal abnormalities. The commonest association is with chromosomal defects. In fetuses with increased NT and a normal karyotype, the risk of an adverse outcome remains and increases with increasing NT. An NT of 3.5 mm or more should be considered significant and warrants further investigations by serum markers, depending upon the gestational age. Patients should be counseled for increased risk of fetal loss before embarking upon any invasive manipulation. Fetal outcome is favorable in the absence of any identified abnormalities and with resolution of NT thickening in the progressive scans. For reproducible and accurate measurements of NT, strict adherence to quality guidelines of the technique, training and supervision of the sonologist is of utmost importance.
a Fetal death in the absence of chromosomal abnormalities.
b Residual rate excluding fetal death, major abnormalities, or chromosomal abnormalities.
[1]. Yoon PW, Olney RS, Khoury MJ, Sappenfield WM, Chavez GF, Taylor D, Contribution of birth defects and genetic diseases to pediatric hospitalizations: a population-based studyArchives of pediatrics & adolescent medicine 1997 151(11):1096 [Google Scholar]
[2]. Souka AP, von Kaisenberg CS, Hyett JA, Sonek JD, Nicolaides KH, Increased nuchal translucency with normal karyotypeAmerican journal of obstetrics and gynecology 2005 192(4):1005-21. [Google Scholar]
[3]. Malone FD, D’Alton ME, First-Trimester Sonographic Screening for Down SyndromeObstetrics & Gynecology 2003 102(5,Part 1):1066-79. [Google Scholar]
[4]. Nevo O, Glanc P, The Role of First-Trimester Anatomy in Obstetrical UltrasoundUltrasound Clinics 2012 7(1):57-73. [Google Scholar]
[5]. Lee P, Won H, Chung J, Shin H, Kim A, The variables affecting nuchal skin fold thickness in mid trimesterPrenatal diagnosis 2003 23(1):60-4. [Google Scholar]
[6]. Scheier M, Egle D, Himmel I, Ramoni A, Viertl S, Huter O, Impact of nuchal cord on measurement of fetal nuchal translucency thicknessUltrasound in obstetrics & gynecology 2007 30(2):197-200. [Google Scholar]
[7]. Taipale P, Ämmälä M, Salonen R, Hiilesmaa V, Learning curve in ultrasonographic screening for selected fetal structural anomalies in early pregnancyObstetrics & Gynecology 2003 101(2):273-8. [Google Scholar]
[8]. Bronshtein M, Zimmer EZ, Prenatal ultrasound examinations: for whom, by whom, what, when and how many?Ultrasound in obstetrics & gynecology 2002 10(1):1-4. [Google Scholar]
[9]. Michailidis G, Economides D, Nuchal translucency measurement and pregnancy outcome in karyotypically normal fetusesUltrasound in obstetrics & gynecology 2002 17(2):102-5. [Google Scholar]
[10]. Sairam S, Carvalho JS, Early fetal echocardiography and anomaly scan in fetuses with increased nuchal translucencyEarly Human Development 2012 88(5):269-72. [Google Scholar]
[11]. Hyett J, Moscoso G, Papapanagiotou G, Perdu M, Nicolaides K, Abnormalities of the heart and great arteries in chromosomally normal fetuses with increased nuchal translucency thickness at 1l—13 weeks of gestationUltrasound in obstetrics & gynecology 1996 7(4):245-50. [Google Scholar]
[12]. Clur S, Ottenkamp J, Bilardo C, The nuchal translucency and the fetal heart: a literature reviewPrenatal diagnosis 2009 29(8):739-48. [Google Scholar]
[13]. Cheng EY, Prenatal Diagnosis: Noninvasive ScreeningUltrasound Clinics 2011 6(1):1-10. [Google Scholar]
[14]. Bekker M, A normal 20 week scan of a euploid fetus with a history of first trimester increased nuchal translucency: caution or reassurance?Ultrasound in obstetrics & gynecology 2007 30(1):8-10. [Google Scholar]
[15]. Westin M, Saltvedt S, Bergman G, Almström H, Grunewald C, Valentin L, Is measurement of nuchal translucency thickness a useful screening tool for heart defects? A study of 16 383 fetusesUltrasound in obstetrics & gynecology 2006 27(6):632-9. [Google Scholar]
[16]. Bahado-Singh RO, Wapner R, Thom E, Zachary J, Platt L, Mahoney MJ, Elevated first-trimester nuchal translucency increases the risk of congenital heart defectsAmerican journal of obstetrics and gynecology 2005 192(5):1357-61. [Google Scholar]
[17]. Syngelaki A, Chelemen T, Dagklis T, Allan L, Nicolaides KH, Challenges in the diagnosis of fetal non chromosomal abnormalities at 11—13 weeksPrenatal diagnosis 2011 31(1):90-102. [Google Scholar]
[18]. Rasiah S, Publicover M, Ewer A, Khan K, Kilby M, Zamora J, A systematic review of the accuracy of first trimester ultrasound examination for detecting major congenital heart diseaseUltrasound in obstetrics & gynecology 2006 28(1):110-6. [Google Scholar]
[19]. Scott F, Boogert A, Sinosich M, Anderson J, Establishment and application of a normal range for nuchal translucency across the first trimesterPrenatal diagnosis 1998 16(7):629-34. [Google Scholar]
[20]. Mogra R, Alabbad N, Hyett J, Increased nuchal translucency and congenital heart diseaseEarly Human Development 2012 88(5):261-7. [Google Scholar]
[21]. Berdahl LD, Wenstrom KD, Hanson JW, Web neck anomaly and its association with congenital heart diseaseAmerican journal of medical genetics 2005 56(3):304-7. [Google Scholar]
[22]. Cicero S, Sacchini C, Rembouskos G, Nicolaides KH, Sonographic Markers of Fetal Aneuploidy—A ReviewPlacenta 2003 24(Supplement 2(0)):S88-S98. [Google Scholar]
[23]. Smith D, First-Trimester Nuchal Translucency Screening to Detect Down SyndromeNewborn and Infant Nursing Reviews 2008 8(1):e1-e6. [Google Scholar]
[24]. Wald N, Rodeck C, Hackshaw A, Rudnicka A, SURUSS in perspectiveSemin Perinatol 2005 29(4):225-35. [Google Scholar]
[25]. Wapner R, Thom E, Simpson JL, Pergament E, Silver R, Filkins K, First-trimester screening for trisomies 21 and 18Obstetrical & gynecological survey 2004 59(3):177 [Google Scholar]
[26]. Stewart TL, Malone FD, First trimester screening for aneuploidy: nuchal translucency sonographySeminars in perinatology 1999 Elsevier [Google Scholar]
[27]. Malone FD, D’Alton ME, First-trimester sonographic screening for Down syndromeObstetrics & Gynecology 2003 102(5):1066-79. [Google Scholar]
[28]. De Biasio P, Siccardi M, Volpe G, Famularo L, Santi F, Canini S, First trimester screening for Down syndrome using nuchal translucency measurement with free hCG and PAPP A between 10 and 13 weeks of pregnancy-the combined testPrenatal diagnosis 1999 19(4):360-3. [Google Scholar]
[29]. Kagan KO, Wright D, Valencia C, Maiz N, Nicolaides KH, Screening for trisomies 21, 18 and 13 by maternal age, fetal nuchal translucency, fetal heart rate, free-hCG and pregnancy-associated plasma proteinA Human Reproduction 2008 23(9):1968-75. [Google Scholar]
[30]. Canick JA, Kellner LH, First trimester screening for aneuploidy: serum biochemical markersSeminars in perinatology 1999 Elsevier [Google Scholar]
[31]. Crossley JA, Aitken DA, Cameron AD, McBride E, Connor JM, Combined ultrasound and biochemical screening for Down’s syndrome in the first trimester: a Scottish multicentre studyBJOG: An International Journal of Obstetrics & Gynaecology 2003 109(6):667-76. [Google Scholar]
[32]. Bindra R, Heath V, Liao A, Spencer K, Nicolaides K, One stop clinic for assessment of risk for trisomy 21 at 11—14 weeks: a prospective study of 15 030 pregnanciesUltrasound in obstetrics & gynecology 2002 20(3):219-25. [Google Scholar]
[33]. Malone FD, Canick JA, Ball RH, Nyberg DA, Comstock CH, Bukowski R, First-trimester or second-trimester screening, or both, for Down’s syndromeNew England Journal of Medicine 2005 353(19):2001-11. [Google Scholar]
[34]. Wald N, Watt H, Hackshaw A, Integrated screening for Down’s syndrome based on tests performed during the first and second trimestersNew England Journal of Medicine 1999 341(7):461-7. [Google Scholar]
[35]. Orlandi F, Bilardo C, Campogrande M, Krantz D, Hallahan T, Rossi C, Measurement of nasal bone length at 11—14 weeks of pregnancy and its potential role in Down syndrome risk assessmentUltrasound in obstetrics & gynecology 2003 22(1):36-9. [Google Scholar]
[36]. Cicero S, Curcio P, Papageorghiou A, Sonek J, Nicolaides K, Absence of nasal bone in fetuses with trisomy 21 at 11—14 weeks of gestation: an observational studyThe lancet 2001 358(9294):1665-7. [Google Scholar]
[37]. Hutchon DJR, Monni G, Hjalgrim H, de Biasio P, Venturini P, Reynolds T, Absence of nasal bone and detection of trisomy 21Lancet (London, England) 2002 359(9314):1343 [Google Scholar]
[38]. Wald N, Rodeck C, Hackshaw A, Walters J, Chitty L, Mackinson A, SURUSS Research Group. First and second trimester antenatal screening for Down’s syndrome: the results of the Serum, Urine and Ultrasound Screening Study (SURUSS)Health Technol Assess 2003 7:1-77. [Google Scholar]
[39]. D’Alton M, Cleary-Goldman J, First and second trimester evaluation of risk for fetal aneuploidy: the secondary outcomes of the FASTER TrialSeminars in perinatology 2005 Elsevier [Google Scholar]
[40]. Hyett J, Brizot M, Von Kaisenberg C, McKie A, Farzaneh F, Nicolaides K, Cardiac gene expression of atrial natriuretic peptide and brain natriuretic peptide in trisomic fetusesObstetrics & Gynecology 1996 87(4):506-10. [Google Scholar]
[41]. Clark EB, Neck web and congenital heart defects: A pathogenic association in 45 X O Turner syndrome?Teratology 2005 29(3):355-61. [Google Scholar]
[42]. Lacro RV, Jones KL, Benirschke K, Coarctation of the aorta in Turner syndrome: a pathologic study of fetuses with nuchal cystic hygromas, hydrops fetalis, and female genitaliaPediatrics 1988 81(3):445-51. [Google Scholar]
[43]. Simpson J, Sharland G, Nuchal translucency and congenital heart defects: heart failure or not?Ultrasound in obstetrics & gynecology 2002 16(1):30-6. [Google Scholar]
[44]. Driscoll DA, Morgan MA, Schulkin J, Screening for Down syndrome: changing practice of obstetriciansAmerican journal of obstetrics and gynecology 2009 200(4):459:e1-e9. [Google Scholar]
[45]. Hyett J, Perdu M, Sharland G, Snijders R, Nicolaides KH, Using fetal nuchal translucency to screen for major congenital cardiac defects at 10-14 weeks of gestation: population based cohort studyBmj 1999 318(7176):81-5. [Google Scholar]
[46]. Goetzl L, Adverse Pregnancy Outcomes After Abnormal First-Trimester Screening for AneuploidyClinics in Laboratory Medicine 2010 30(3):613-28. [Google Scholar]
[47]. Spencer K, Accuracy of Down syndrome risks produced in a first-trimester screening programme incorporating fetal nuchal translucency thickness and maternal serum biochemistryPrenatal diagnosis 2002 22(3):244-6. [Google Scholar]
[48]. Ghi T, Huggon I, Zosmer N, Nicolaides K, Incidence of major structural cardiac defects associated with increased nuchal translucency but normal karyotypeUltrasound in obstetrics & gynecology 2001 18(6):610-4. [Google Scholar]
[49]. Mavrides E, Cobian Sanchez F, Tekay A, Moscoso G, Campbell S, Thilaganathan B, Limitations of using first trimester nuchal translucency measurement in routine screening for major congenital heart defectsUltrasound in obstetrics & gynecology 2001 17(2):106-10. [Google Scholar]
[50]. Petrikovsky BM, Baker D, Schneider E, Fetal hydrops secondary to human parvovirus infection in early pregnancyPrenatal diagnosis 1999 16(4):342-4. [Google Scholar]
[51]. Bilardo C, Pajkrt E, De Graaf I, Mol B, Bleker O, Outcome of fetuses with enlarged nuchal translucency and normal karyotypeUltrasound in obstetrics & gynecology 2002 11(6):401-6. [Google Scholar]
[52]. Markov D, Jacquemyn Y, Leroy Y, Bilateral cleft lip and palate associated with increased nuchal translucency and maternal cocaine abuse at 14 weeks of gestationClinical and experimental obstetrics & gynecology 2003 30(2-3):109 [Google Scholar]
[53]. Clementschitsch G, Hasenohrl G, Steiner H, Staudach A, Early diagnosis of a fetal skeletal dysplasia associated with increased nuchal translucency with 2D and 3D ultrasoundUltraschall in der Medizin 2003 24(5):349-52. [Google Scholar]
[54]. Geipel A, Berg C, Germer U, Krapp M, Kohl M, Gembruch U, Mucopolysaccharidosis VII (Sly disease) as a cause of increased nuchal translucency and non immune fetal hydrops: study of a family and technical approach to prenatal diagnosis in early and late pregnancyPrenatal diagnosis 2002 22(6):493-5. [Google Scholar]
[55]. Sharp P, Haan E, Fletcher J, Khong TY, Carey W, first trimester diagnosis of smith—lemli—opitz syndromePrenatal diagnosis 1998 17(4):355-61. [Google Scholar]
[56]. Hiippala A, Eronen M, Taipale P, Salonen R, Hiilesmaa V, Fetal nuchal translucency and normal chromosomes: a long term follow up studyUltrasound in obstetrics & gynecology 2002 18(1):18-22. [Google Scholar]
[57]. Scholl J, Iyer C, Heard A, Coletta J, Panda B, Russell M, 415: Thicker nuchal translucency is associated with greater risk of abnormal fetal and neonatal outcomes among fetuses with first trimester cystic hygromaAmerican Journal of Obstetrics and Gynecology 2011 204(1, Supplement):S168 [Google Scholar]