INTRODUCTION
Perhaps no other anatomical feature more closely epitomizes vertebrates than the head. Being comprised of paired sensory elements, a muscularized masticatory apparatus, and a cartilaginous or bony braincase, the head develops from the complex and massive movements of the mesenchymal cells which are derived from the mesoderm and the neural crest [1]. These cells interact with each other and with a variety of craniofacial epithelia to produce the intricate structures of the face, skull, teeth, and the jaw.
Craniofacial abnormalities account for up to one-third of all the congenital birth defects and many of these anomalies are thought to arise as a result of perturbations in the neural crest cell formation, distribution (migration?), growth and/or differentiation. The complexity of the interactions that occur between the neural crest cells and other cranial tissues during the head development, demonstrates that many of these abnormalities may also occur due to primary defects in the paraxial mesoderm, ectoderm or endoderm tissues with which the neural crest cells interact [2].
We are reporting one such case of a stillborn foetus with complete absence of the facial morphology and the neck structures. To the best of our knowledge as per a review of the available literature, it seems that such a case has not been described previously.
CASE REPORT
A stillborn, full term, vaginally delivered, male foetus was brought to our museum from the Department of Paediatrics. His parents gave a history of a miscarriage in his mother’s first pregnancy. There was no history of any drug intake. She had received limited antenatal care in a primary health centre, where no antenatal ultrasonography had been carried out. Her subsequent gestation period was uneventful. There was no history of anyone else in her family suffering from such a defect.
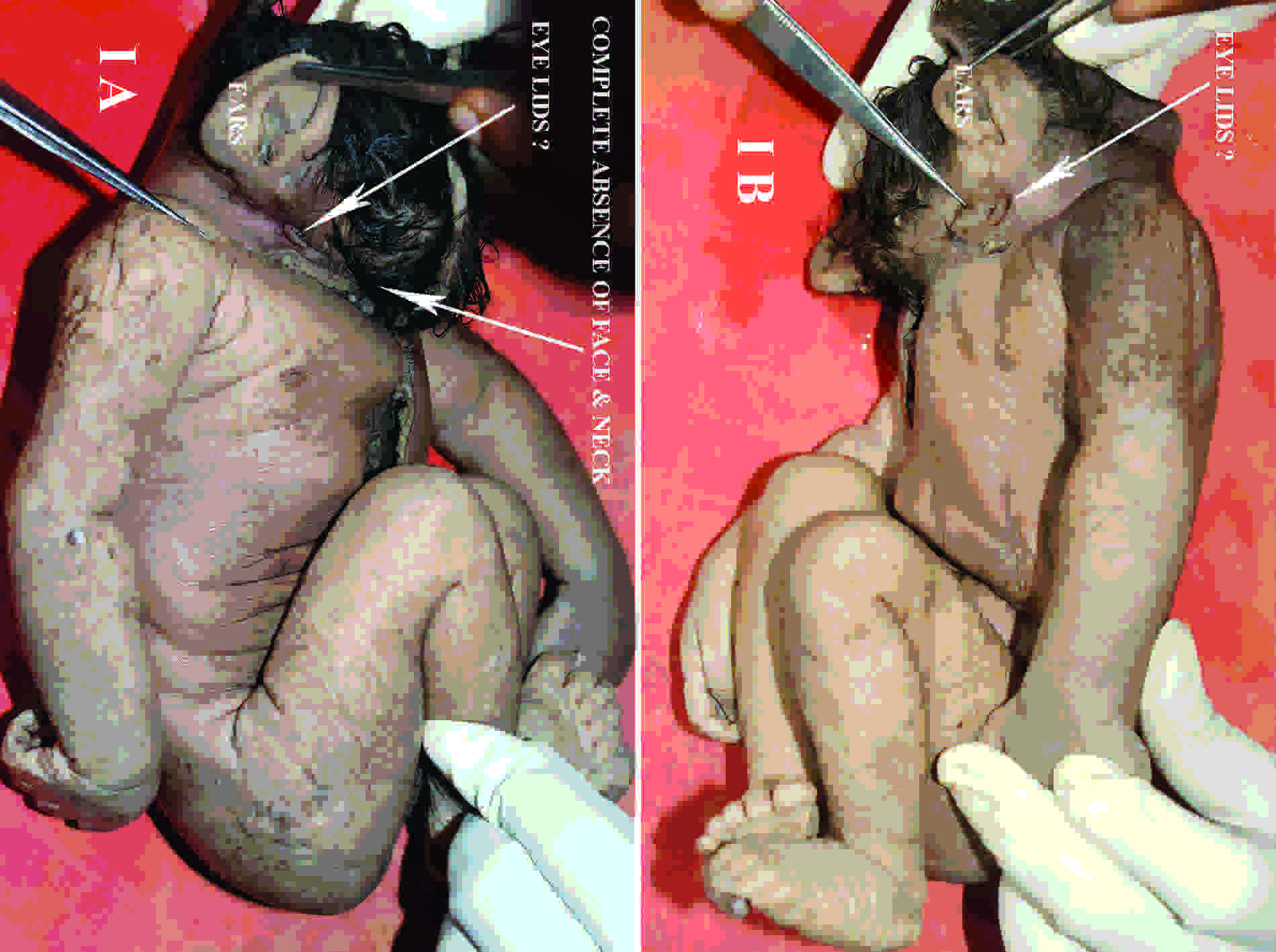
The gross examination revealed absence of the face (frontonasal area and maxillary and mandibular features) and neck portions in an otherwise morphologically normal foetus. The hair growth was abundant and it was present all over the occipital region, just above the ears, and in the frontal region, till the junction between the head and the thorax.
Both the ears were well developed for the foetal age and they were positioned at their normal locations. Close to the junction between the head and the thorax, underdeveloped eyelid-like structures were seen on both sides, with no orbital cavity and its contents [Table/Fig-1]. A micropenis was noted with median scrotal raphe and rugosity, but the gonads appeared as small grayish white structures with no obvious testicular microarchitecture. No congenital anomalies were noted in the limbs.
(A) Photograph of fetus in right lateral view & (B) in left lateral view, showing absence of face and neck structures, well developed ears and underdeveloped eyelids with no eyeballs
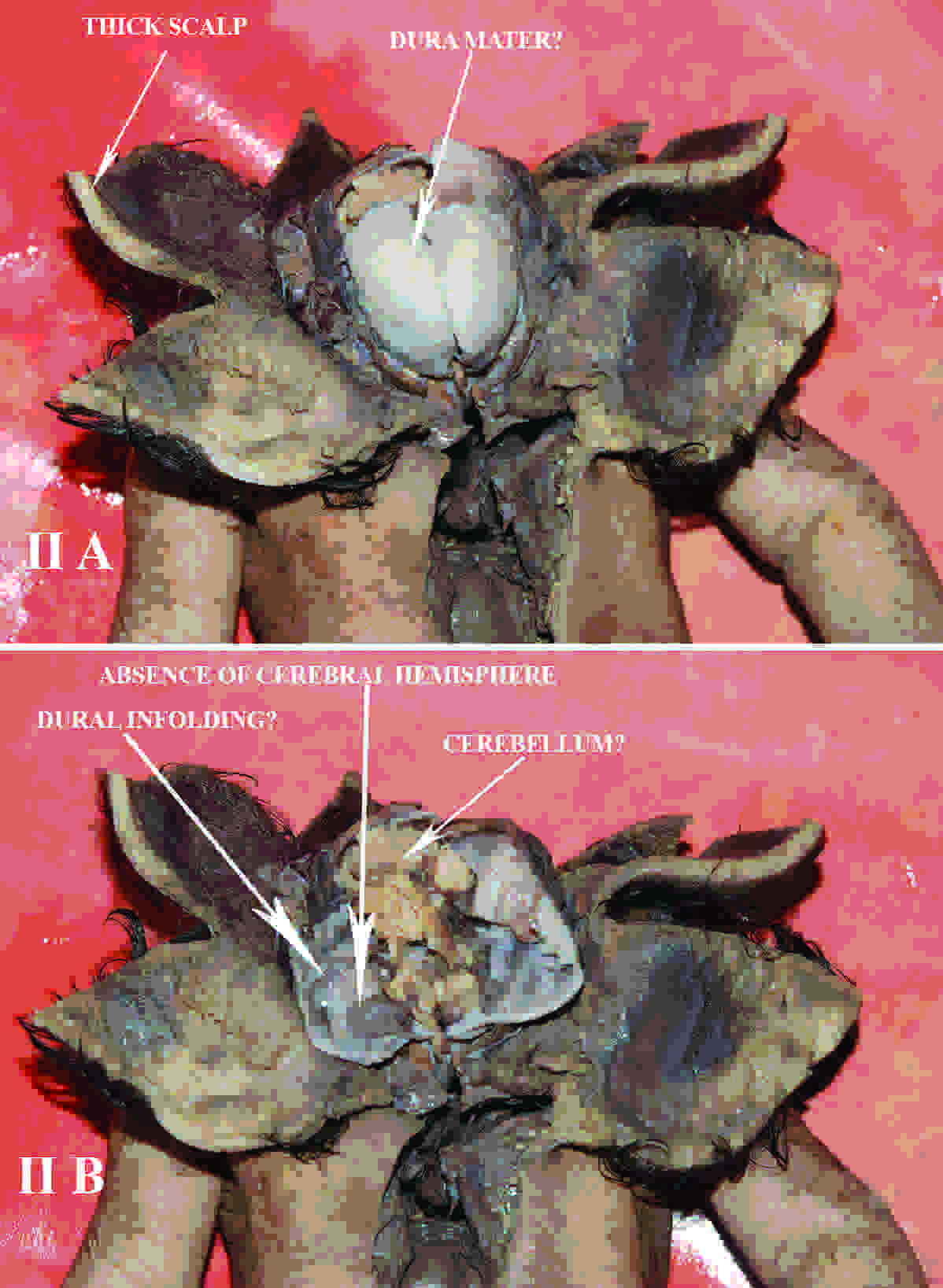
The scalp was 0.7 cm thick and a glistening, membrane like sheath was found beneath it, which could represent the cranial vault. The interior of the cranial vault [Table/Fig-2] presented an ill defined cerebellar foliation like morphology in its posterior part, with no visible cerebrum or dura and their venous sinuses, the pituitary fossa and its gland and cranial nerves and foraminas in the cranial fossa.
(A) Photograph of dissected fetus showing thick scalp and membranous skull bones and (B) depicting interior of skull
Grossly, the thoracic and abdominal organ development was normal for the foetal age, with no definable anomalies. Upon interior dissection of the heart, the two atria were found to have a wide communication, which could have been a wide patent foramen ovale. Also, an oval shaped gap existed in the membranous interventricular septum, with hypoplastic papillary muscles in both the ventricles. The branching pattern of the arch of the aorta was normal.
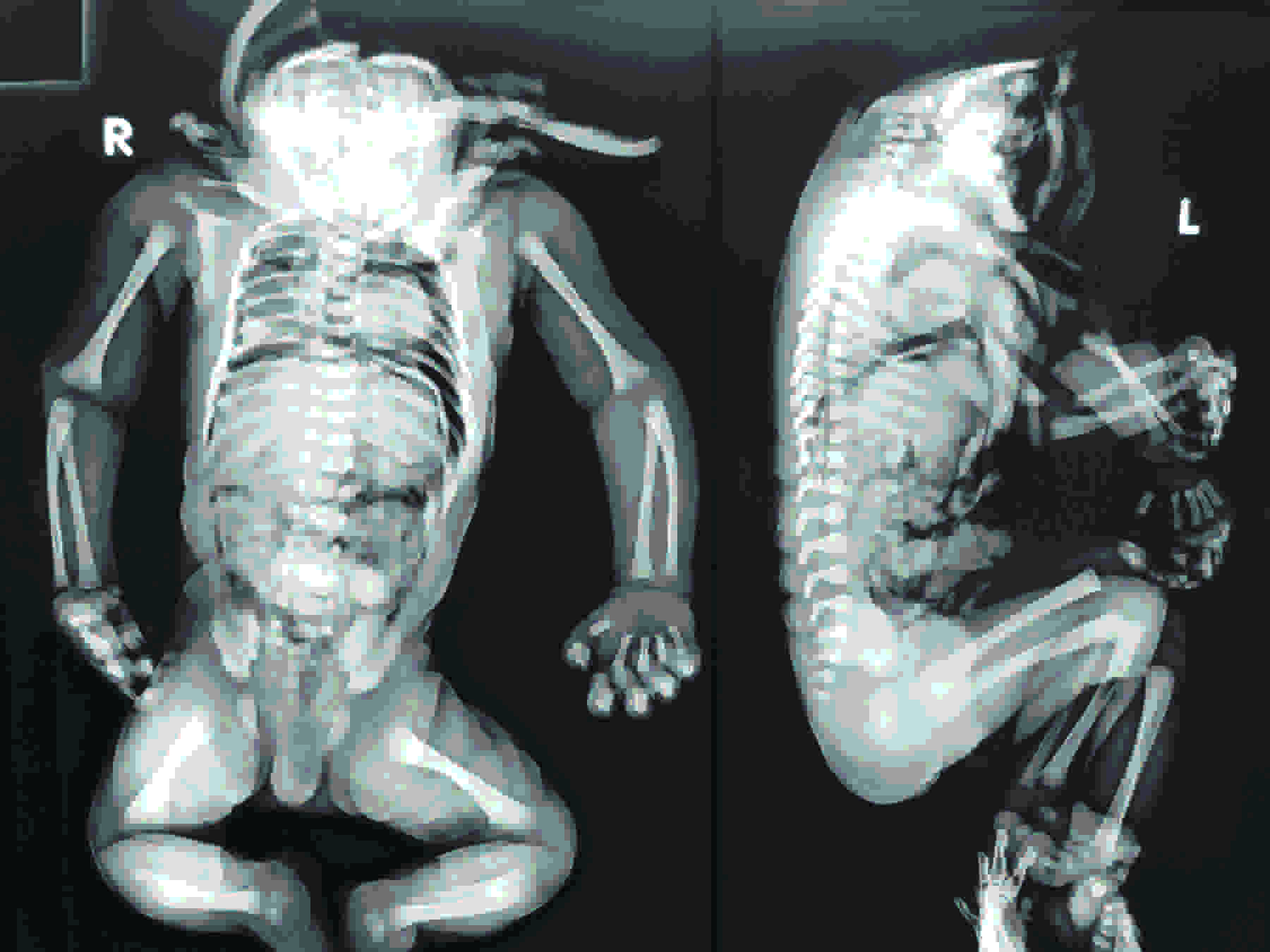
The babygram [Table/Fig-3] showed a cranial vault with complete absence of the facial skeleton, the hyoid bone, and the thyroid cartilages. The vertebral column was normal in terms of the number of vertebral bodies and their articulation. A set of ribs which were normal in morphology were seen, which had an articulation with the corresponding vertebral bodies. No skeletal anomalies were noted in the limbs.
Infantogram of the fetus born with absent face and neck
DISCUSSION
A uniform hypothesis regarding the aetiology of craniofacial anomalies remains unknown because of the paucity of available details.
The neural crest cells display a highly stereotyped migratory pattern, passing between the neural and the facial epithelia, and around the paraxial mesoderm, until they reach their locations in the pharyngeal arches and the frontonasal process. As these ectodermally derived cells migrate, they contribute extensively to the formation of the mesenchymal structures in the head and neck [1].
The Cranial Neural Crest (CNC) cells interact with and are consequently instructed by the pharyngeal endoderm, the branchial arch ectoderm, and the isthmic organizer at the midbrain—hindbrain boundary [3], before giving rise to various types of tissues such as the bone, cartilage, tooth, and the cranial nerve ganglia in the craniofacial region [4–6].
The paraxial mesoderm populations which are located super-ficially beside the hindbrain are a source of the branchial arch and the associated musculature [7,8]. These include the jaw closing (1st arch) and opening (2nd arch) muscles, the facial muscles, and the hyobranchial muscles which are innervated by the trigeminal, facial and the glossopharyngeal nerves. All the head muscle primordia exit from their initial niches in the paraxial mesoderm and they move into the periocular and the branchial locations which are populated by the connective tissue progenitors which are derived from the neural crest [5,9]. The only exceptions are the progenitors of the tongue muscles.
In mammals, both the CNC- and the mesoderm-derived cells contribute to the development of the skull. The skull consists of the neurocranium and the viscerocranium. The neurocranium (skull vault and base) surrounds and protects the brain. The viscerocranium (the jaws and other pharyngeal arch derivatives) which is derived from the neural crest, forms the face. Couly and co-workers concluded that the skull vault was entirely neural crest derived [10]. It was demonstrated by Jiang et al., that the frontal bones were neural crest-derived and that the parietal bones were of mesoderm origin [11]. The posterior part of the skull vault is derived from the occipital somites. Later, as the cerebral vesicles expand beneath the frontal and the parietal elements, the crest-derived, meningeal cells which surround these parts of the brain are brought beneath the frontal and the parietal osteogenic tissues, to form the dura. The dura mater that underlies the frontal and parietal bones is also neural crest derived [11,12].
In the upper and middle face, the structures which are derived from the frontonasal process and the Sonic hedgehog (Shh) and the FGF signaling, appear to be critical for setting up a boundary in the neural and the surface ectoderm [13]. The endothelin-mediated Gsc expression is critical for controlling the patterning of the mandible [14]. Mutations in either Endothelin-1 or Gsc result in mandibular development defects. A conditional inactivation of Bmpr1a in the oral ectoderm, results in tooth agenesis [1], thus demonstrating the significance of the BMP signaling in the patterning of the craniofacial structures.
The pharyngeal pouches generate several specialized epithelial structures such as the thyroid, the parathyroid, and the thymus [4]. The development of these organs involves the interaction between the pharyngeal endoderm and its flanking cranial neural crest cells. A compromised retinoic acid signaling from the pharyngeal mesoderm affects the development of the pharyngeal endoderm, which in turn causes defects in the CNC migration and in the development of the pharyngeal pouch-derived organs such as the thymus and the parathyroid glands [5].
Le Dourain and Le Lievre demonstrated that the NC contribute to the meninges of the forebrain and that when they are absent, the forebrain undergoes apoptosis, as the head mesenchyme cannot compensate for the loss of the NCC [15]. Thus, NCC has an evolutionary significance in the development of the primitive brain and in the growth of the forebrain.
From the results which have been presented here, together with those of other authors, it appears that the neural crest cells display a highly stereotyped migratory pattern, passing between the neural and the facial epithelia, and around the paraxial mesoderm until they reach their locations in the pharyngeal arches and in the frontonasal process. Any inaccurate or interrupted migration can lead to a craniofacial malformation. Precisely, how these disruptions influence the cellular interaction and the skeletal defects, is not clear.
CONCLUSION
In conclusion, the cephalic neural crest cells interact with and are consequently instructed by pharyngeal endoderm, the ectoderm, and the mesoderm, before giving rise to various types of tissues in the craniofacial regions. There are no “craniofacial genes” as such. The same morphogen (BMP, TGF, FGF, Shh, and so on) that controls the limb development also plays a crucial role in regulating the craniofacial morphogenesis.
Once one knows these cranial specific pathways, then he/she might focus on how to prevent or at least mitigate the cranial skeletal anomalies. Such a research will have direct and profound implications in the treatment of cranial skeletal defects which result from malformations.
[1]. Chai Y, Maxson RE, Recent advances in craniofacial morphogenesisDevelopmental Dynamics 2006 235:2353-75. [Google Scholar]
[2]. Noden DM, The embryonic origins of avian cephalic and cervical muscles and associated connective tissuesAm J Anat 1983 168:257-76. [Google Scholar]
[3]. Trainor PA, Krumlauf R, Patterning the cranial neural crest: hind- brain segmentation and Hox gene plasticityNat Rev Neurosci 2000 1:144-65. [Google Scholar]
[4]. Graham A, Okabe M, Quinlan R, The role of the endoderm in the development and evolution of the pharyngeal archesJ Anat 2005 207:479-87. [Google Scholar]
[5]. Noden DM, The role of the neural crest in patterning of avian cranial skeletal, connective, and muscle tissuesDevel Biol 1983 96:144-65. [Google Scholar]
[6]. Noden DM, Trainor PA, Relations and interactions between cranial mesoderm and neural crest populationJ Anat 2005 207:575-601. [Google Scholar]
[7]. Niederreither K, Vermot J, Le Roux I, Schuhbaur B, Chambon P, Dolle P, The regional pattern of retinoic acid synthesis by RALDH2 is essential for the development of posterior pharyngeal arches and the enteric nervous systemDevelopment 2003 130:2525-34. [Google Scholar]
[8]. Trainor PA, Tam PPL, Cranial paraxial mesoderm and neural crest of the mouse embryo-codistribution in the craniofacial mesenchyme but distint segregation in the branchial archesDevelopment 1995 121:2569-82. [Google Scholar]
[9]. Trainor PA, Sobieszczuk D, Wilkinson D, Krumlauf R, Signalling between the hindbrain and paraxial tissues dictates neural crest migration pathwaysDevelopment 2002 129:433-42. [Google Scholar]
[10]. Couly GF, Coltey PM, Le Douarin NM, The triple origin of skull in higher vertebrates: a study in quail-chick chimerasDevelopment 1993 117:409-29. [Google Scholar]
[11]. Jiang X, Iseki S, Maxon RE, Sucov HM, Morris-Kay GM, Tissue origins and interactions in the mammalian skull vaultDev Biol 2002 241:106-10. [Google Scholar]
[12]. Ito Y, Yeo JY, Chytil A, Han J, Bringas P, Nakajima A, Conditional inactivation of Tgfbr2 in cranial neural crest causes cleft palate and calvaria defectsDevelopment 2003 130:5269-80. [Google Scholar]
[13]. Helms JA, Cordero D, Tapadia MD, New insights into craniofacial morphogenesisDevelopment 2005 132:851-61. [Google Scholar]
[14]. Clothier DE, Hosoda K, Richardson JA, Williams SC, Yanagisawa H, Kuwaki T, Kumada M, Hammer RE, Yanagisawa M, Cranial and cardiac neural crest defects in endothelin-A receptor deficient miceDevelopment 1998 125:813-24. [Google Scholar]
[15]. Le Lievre CS, Le Douarin NM, Mesenchymal derivatives of the neural crest: analysis of ckimeric quail and chick embryosJ Embryol Exp Morphol 1975 34:125-54. [Google Scholar]