Livedoid Vasculopathy and Mononeuritis Multiplex, with a Fulminant Hepatic Failure which was caused by Herpes Simplex Hepatitis: A Case Report
Sathish Pai B1, Kanthilatha Pai2
1 Professor, Department of Dermatolology, Kasturba Medical College, Manipal University, India.
2 Professor, Department of Pathology, Kasturba Medical College, Manipal University, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Sathish Pai B, Professor, Department of Dermatology, Kasturba Medical College, Manipal University, India.
Phone: 09900405072
E-mail: drsbpai@yahoo.co.in
Livedoid vasculopathy with mononeuritis multiplex is a rare association. We are presenting a case of an unusual association of livedoid vasculopathy with mononeuritis multiplex, who developed fulminant hepatic failure which was secondary to Herpes simplex virus (HSV) hepatitis, while she was on treatment with immunosuppressants. Her skin biopsy and immunofluorescence studies showed the features of vasculitis. A biopsy from the sural nerve showed the features of chronic vasculitis.
Livedoid vasculopathy, Mononeuritis multiplex, Herpes simplex hepatitis
INTRODUCTION
Livedoid vasculopathy (LV) is a rare, vasculopathic disorder which manifests as recurrent, painful, reticulated, and ulcerative lesions of the legs, which result in ivory atrophic scars with peripheral telangiectasia and hyperpigmentation. It predominantly affects females. Although the typical dermatological findings and the pathogenesis of this condition have been emphasized previously, the involvement of peripheral neuropathy with this dermatological vasculopathy is uncommon [1–3]. Mononeuritis multiplex is defined as a disorder which involves two or more named peripheral nerve trunks and which occurs most often in collagen vascular disorders or as a manifestation of non- systemic vasculitis which is restricted to the peripheral nerves [4].
CASE REPORT
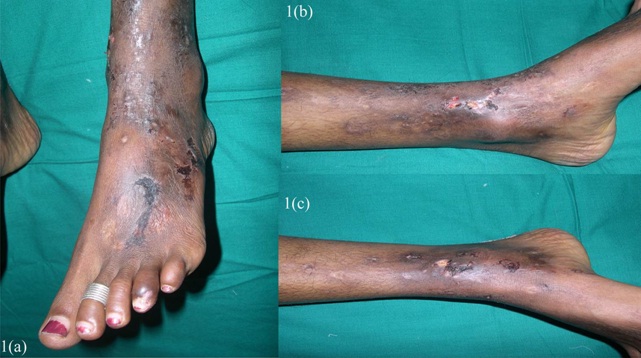
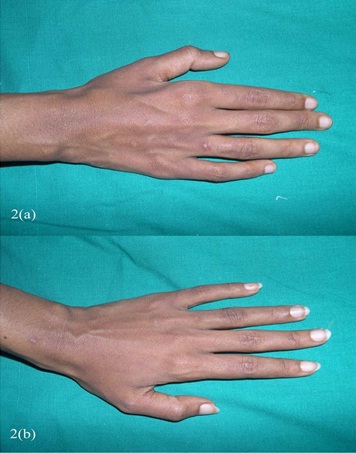
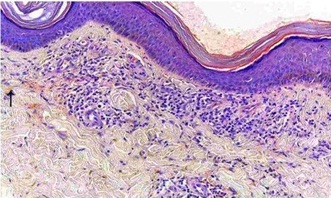
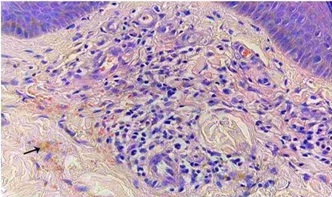
A 27 year old female presented with recurrent painful ulcers over the ankles and the adjacent dorsum of the feet, of 2 year’s duration. She also complained of muscle weakness in both her hands. She noticed the development of the painful purpuric lesions which later formed painful ulcers. She gave no history of recurrent pedal oedema, trauma; dilated veins over the legs, photosensitivity or any systemic symptoms which were suggestive of connective tissue disorders. There was no history of vesicles, pustules and painful nodules. The patient’s family and social histories were unremarkable. There was no drug history. On clinical examination, it was found that there were multiple ulcers, erosions, scars and purpuric macules over the dorsum of the feet and on both ankles [Table/Fig-1a, b, c]. She did not have any hypopigmented anaesthetic patches or peripheral nerve thickening. No systemic manifestations or features of vascular insufficiency were present. Her blood pressure was normal. Her neurological examination revealed distal motor weakness of the upper limbs and a graded sensory loss in the lower limbs. There was an asymmetric weakness of the small muscles of the hand, which involved the palmar and the dorsal interossei and a weakness of the abductor pollicis muscle [Table/Fig-2a, b]. A clinical diagnosis of leg ulcers which were secondary to vasculitis was considered and the patient was investigated. In view of the weakness of the small muscles of the hands and the graded sensory loss over the lower limbs, a sural nerve biopsy was done, to rule out Hansen’s disease. Her complete blood count, liver function test, renal function test, blood sugar and urine analyses and chest X-ray were within normal limits. The antinuclear antibodies (ANA) and anticardiolipin antibodies were negative. The serological tests for syphilis, the human immunodeficiency virus and hepatitis B were negative. The arterial and venous Doppler scans of the legs were normal. The histopathological examination revealed occasional intravascular thrombi, extravasation of the RBCs and a perivascular lymphocytic infiltrate [Table/Fig-3a,b]. Immunoflorescence showed C3 and fibrin positivities within the dermal vessels in the papillary dermis [Table/Fig-4a,b]. A nerve biopsy (right sural) which was done, showed the features of chronic vasculitis with a marked sectorial fibre loss and chronic axonopathy, with the epineural vessels showing a recanalized lunula. Nerve conduction studies revealed axonal motor sensory asymmetric neuropathy. A final diagnosis of livedoid vasculopathy with mononeuritis multiplex was made.
Showing multiple ulcers, erosions, scars and purpuric macules over dorsum of feet and both ankles.
Shows asymmetric weakness of small muscles of hand.
Photomicrograph showing perivascular lymphocytic infiltrate, extravasated RBC.
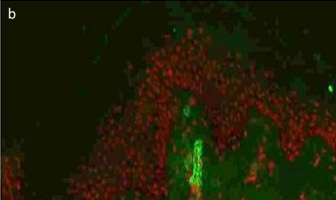
Photomicrograph showing strong fibrin positivity in dermal vessels (DIF, 200X).
Photomicrograph showing strong C3 positivity in dermal vessels (DIF, 200X)
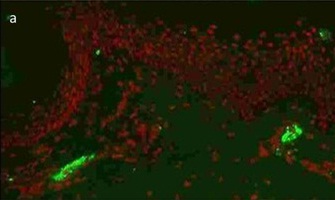
Photomicrograph showing strong fibrin positivity in dermal vessels (DIF, 200X)
She was treated with Prednisolone 40mg per day, azathioprine 50mg per day and pentoxyfylline 400mg three times a day. A physiotherapist’s advice was also sought for the wasting of her hand muscles. Her condition improved after 4 weeks, with complete healing of the ulcers over her legs [Table/Fig-5a, b], but the weakness and wasting of the hand muscles persisted. Both prednisolone and azathioprine were continued as per the advice of the neurologist. Six months later, she developed pain in the toes and a bluish discolouration that aggravated on cold exposure. She did not have ulcers over her legs. The patient was started on Nifedipine, aspirin and pentoxyfylline; the steroids and azathioprine were continued. Despite this treatment, the cyanosis progressed to digital gangrene which involved the 3rd and 4th toes of the left foot, for which surgical amputations were performed. Two weeks after the surgery, the patient developed low grade fever and mild jaundice. The laboratory results showed mild hyperbilirubinaemia (direct bilirubin 2.3mg/dl) and raised liver transminases. (AST-9928, ALT-5502) Her condition progressed to a profound hepatic failure with coma and encephalopathy, resulting in the mortality of the patient. A post-mortem liver biopsy revealed massive necrosis and Herpes simplex hepatitis.
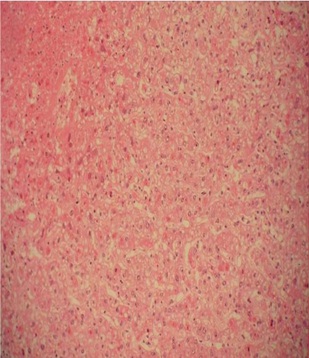
Showing massive liver cell necrosis, H&E 100X.
Showing intranuclear cowdry inclusions H&E 200X.
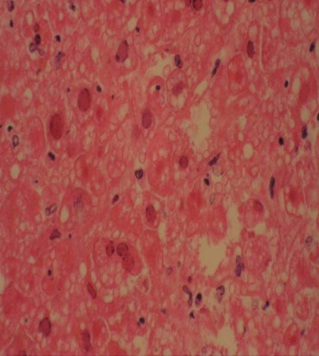
DISCUSSION
Livedoid vasculopathy, an affection which was first described by Milian in 1929 as ‘atrophie blanche’, is a chronic disease [5]. LV was originally described as a clinical manifestation of vasculitis. However, at present, the main physiopathogenic mechanism which is considered is a vaso-occlusive phenomenon which is caused by intraluminal thrombosis of the dermal venules [6].
The histopathological findings of LV are characterized by occlusion of the dermal blood vessels due to intravascular fibrin deposition and thrombosis, segmental hyalinization and endothelial proliferation. There is minimal perivascular lymphocytic infiltration. Direct immunofluorescence usually demonstrates the deposition of immunoglobulin, fibrin and complement components [7].
The differential diagnosis includes cutaneous small vessel vasculitis and cutaneous polyarteritis nodosa (especially when there are symptoms of mononeuritis multiplex), in addition to the antiphospholipid syndrome, chronic venous stasis, pyoderma gangrenosum, factitious dermatitis and Degos disease.
The weakness and wasting of the small muscles of the hands with a graded sensory loss over lower limbs, is a classic feature of mononeuritis multiplex. This was supported by the nerve conduction studies and the findings of the sural biopsy. Many systemic diseases can be related to mononeuritis multiplex, such as vasculitis, connective tissue disease, cryobulinaemia, sarcoidosis, diabetes, amyloidosis, neoplasm, and infections. Our patient did not have systemic features of vasculitis, and the laboratory results did not support the presence of any connective tissue diseases or vasculitis.
The management of livedoid vasculopathy can be challenging. Several therapeutic approaches have been employed, with varying degrees of success. All forms of treatment which were proposed for LV were based on the reports of isolated cases or case series. The therapeutic modalities include antiplatelet agents (aspirin, dipyridamole), fibrinolytic agents (danazol), anticoagulants (subcutaneous heparin, warfarin), vasodilating agents (nifedipine, nicotinic acid), anti-inflammatory agents (prednisone), intravenous immunoglobulin, cyclosporine, hyperbaric oxygen, and pentoxifylline, which may alter the blood viscosity and the erythrocyte flexibility [8]. Leg elevation, compression therapy, bed rest, and topical wound care may aid the healing.
There are only few reported cases of livedoid vasculopathy which had presented in combination with peripheral neuropathy. The involvement of the peripheral nervous system possibly occurs due to areas of multifocal ischaemia, which result from the deposition of fibrin and thrombin in the vasa nervorum. A combination therapy with oral prednisolone and azathioprine has been used in the treatment of livedoid vasculopathy [9]. Gan EY et al., in their retrospective analysis, found the above combination to be useful in the treatment of livedoid vasculopathy, next only to a combination of prednisolone, colchicine and pentoxyfylline [9].
Herpes simplex hepatitis most commonly occurs in immunocompromised patients. In our patient, the treatment with corticosteroids and azathioprine must have produced an immunocompromised state. Clinicians should have a high index of suspicion for HSV hepatitis in immunocompromised patients with elevated liver enzymes and no other underlying cause. Acyclovir treatment should be instituted early in the cases of hepatitis of unknown aetiologies, as an early initiation of this therapy is imperative to prevent a severe disease which can result in liver failure [10]. We have reported an interesting case of livedoid vasculopathy with mononeuritis multiplex that developed Herpes simplex induced hepatitis due to immunosuppression. Only few cases of livedoid vasculopathy with mononeuritis multiplex have been reported in the literature.
[1]. Winkelmann RK, Schroeter AL, Kierland RR, Ryan TM, Clinical studies of livedoid vasculitis: (segmental hyalinizing vasculitis)Mayo Clin Proc 1974 49:746-50. [Google Scholar]
[2]. Toth C, Trotter M, Clark A, Zochodne D, Mononeuropathy multiplex in association with livedoid vasculitisMuscle Nerve 2003 28:634-39. [Google Scholar]
[3]. Osada S, Kimura Y, Kawana S, Case of livedoid vasculopathy with peripheral neuropathy successfully treated with low-dose warfarinJ Dermatol 2010 37:98-101. [Google Scholar]
[4]. Chalk CH, Dyck PJ, Conn DL, Vasculitic neuropathy. In: Dyck PJ, Thomas PK, editorsPeripheral Neuropathy 1993 3rd editionPhiladelphiaW B Saunders:1424-36. [Google Scholar]
[5]. Busam KJ, Barksdale SK, Barnhill RL, Vasculitis and related disorders. In:Barnhill RL, editorsTextbook of Dermatopathology 1998 1961st editionNew YorkMcGraw-Hill [Google Scholar]
[6]. Jee-Eun Kim, Su-Yeon Park, Dong In Sinn, Sung-Min Kim, Yoon-Ho Hong, Kyung Seok Park, Ischemic neuropathy Associated with Livedoid VasculitisJ Clin Neurol 2011 7:233-36. [Google Scholar]
[7]. Khenifer S, Thomas L, Balme B, Dalle S, Livedoid vasculopathy: thrombotic or inflammatory disease?Clin Exp Dermatol 2009 35:693-98. [Google Scholar]
[8]. Criado PR, Rivitti EA, Sotto MN, Valente NYS, Aoki V, Carvaho JF, Livedoid Vasculopathy: an intringuing cutaneous diseaseAn Bras Dermatol 2011 86:961-97. [Google Scholar]
[9]. Gan EY, Tang MBY, Tan SH, Chua SH, Tan AWH, A ten year retrospective study on livedo vasculopathy in Asian patientsAnn Acad Med Singapore 2012 41:400-06. [Google Scholar]
[10]. Klein NA, Mabie WC, Shaver DC, Latham PS, Adamec TA, Pinstein ML, Riely CA, Herpes simplex virus hepatitis in pregnancy. Two patients successfully treated with acyclovirGastroenterology 1991 100:239-44. [Google Scholar]