Primary Urachal Mucinous Adenocarcinoma of the Urinary Bladder
Iqbal Singh1, Ravi Prasad2
1 Consultant Urologist, Professor, Department of Surgery (Urology Div), University College of Medical Sciences (University of Delhi) & GTB Hospital, Delhi-110095.
2 Post graduate Junior Resident, Department of Surgery, University College of Medical Sciences (University of Delhi) & GTB Hospital, Delhi-110095.
NAME, ADRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Iqbal Singh, Consultant Urologist, Professor, Department of Surgery, UCMS (Univ of Delhi) & GTBH, F-14 NDSE-2, New Delhi-110049. India
Phone: 9810499222
E-mail: iqbalsinghp@yahoo.co.uk
Urinary bladder cancer is the second most frequent tumour of the genitourinary tract with bladder adenocarcinoma comprising for about 0.5-2% of all malignant bladder tumours. Other primary sites for such tumours include rectum, stomach, endometrium, breast, prostate, seminal vesicles and ovaries. Such non-urothelial bladder tumours with intramural bladder tumour growth may delay the onset of symptoms which may lead to a delay in the diagnosis and thereby adversely affecting the prognosis as compared to urothelial bladder tumours. Traditionally bladder adenocarcinomas were believed to be resistant to both chemotherapy and radiotherapy, but recent advancements have shown encouraging responses with adjuvant chemotherapy and radiotherapy. We present here a case of primary urachal mucinous adenocarcinoma of the urinary bladder highlighting their relative rarity of occurrence and the difficulties encountered in diagnosing primary bladder mucinous adenocarcinoma.
Adenocarcinoma urinary bladder, Urachal adenocarcinoma, Cancer bladder
CASE HISTORY
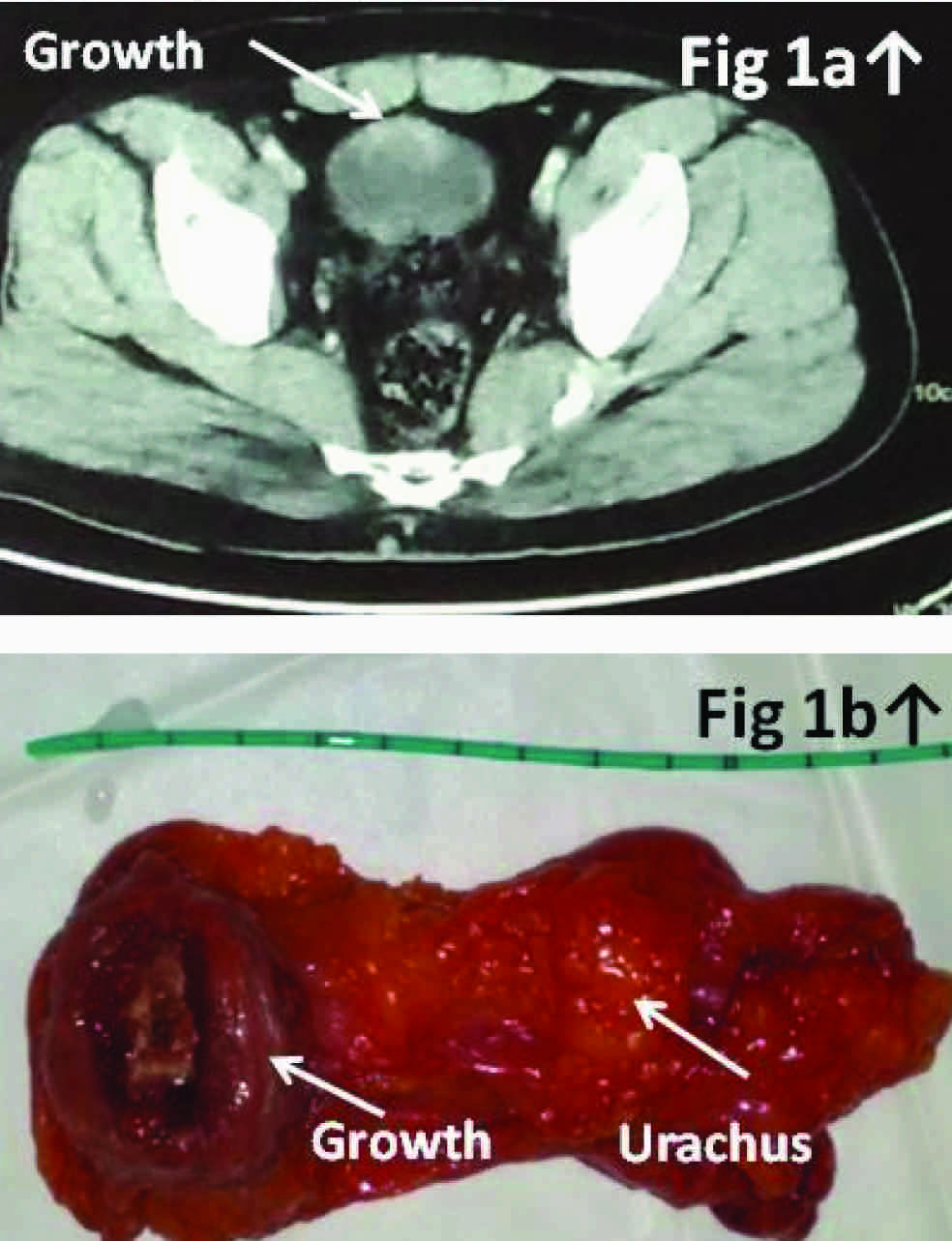
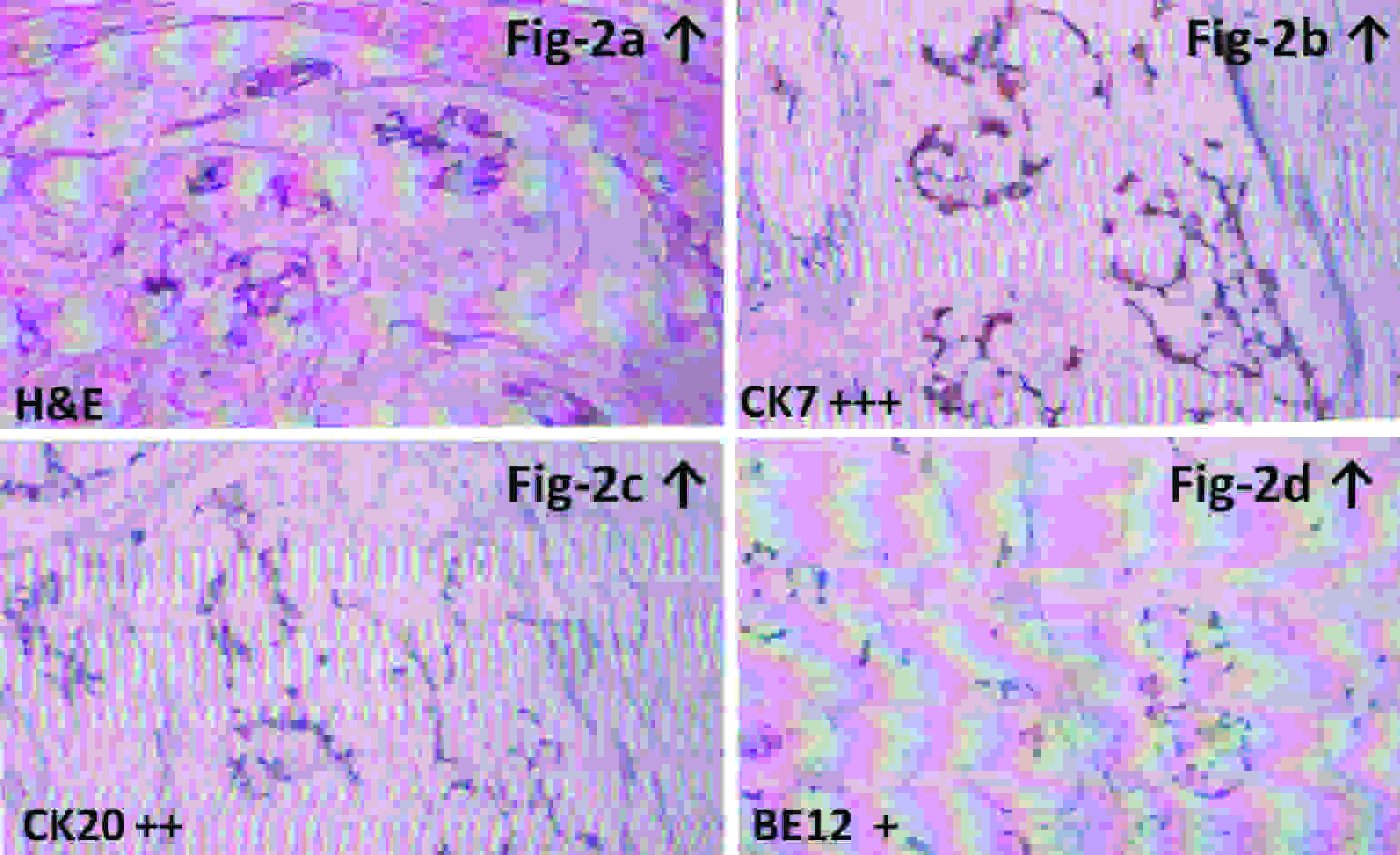
A 40-year old non smoking, gentleman was admitted with complaints of gross intermittent painless hematuria for the preceding two months without any history of anorexia or weight loss. The general physical and urological/digital rectal examination was unremarkable. Urine cytology did not reveal any cytological atypia. Abdominal ultrasonography suggested a distended urinary bladder with irregular mass lesion of size about 2.60x2.27x2.94 cm projecting from the bladder dome along with a midline cystic lesion measuring 13.3x9.4 mm abutting the dome of urinary bladder from outside (suspected urachal cyst) without any peritoneal free fluid or signs of distant metastasis. A contrast enhanced computed tomography (CECT) of abdomen revealed a well defined partly cystic mass with wall enhancement and inhomogeneous contents projecting into the urinary bladder about 3.5 cms in size along with an extravesical mass about 2.5 cm protrusion in size [Table/Fig-1] with vesical fascia present on either side of the latter lesion confirming extraperitoneal localization. The mass was located along the mid anterior surface of the bladder and extends partly towards the umbilicus without any regional infiltration/metastasis (suspected urachal malignancy). PET CT images confirmed presence of a hypermetabolic soft tissue density lesion arising from the junction of antero-inferior and postero-superior bladder wall along with perivesical invasion suggesting an advanced urachal bladder tumor (T3NoMo Stage–III). Diagnostic cystopanendoscopy revealed a sessile ulceroproliferative solid lesion in the dome of the bladder with bilateral normal ureteric orifices. The patient underwent an uneventful transurethral resection of the bladder tumor and his histopathology was reported as transitional epithelial lining with squamous metaplasia, lamina infiltration with well formed glands lined by mucus secretory columnar epithelium with mild to moderate pleomorphism and occasional mitosis along with areas of papillary formation and large pools of extracellular mucin in the background with intracellular mucin with signet ring cells, suggestive of an invasive bladder adenocarcinoma. After informed counseling the patient opted for an umbilical sparing open partial cystectomy with en-bloc excision of the urachal growth. The gross specimen revealed a polypoidal growth 3×3×2 cm on the luminal side with perivesical cystic mass extending perivesically into the urachus. The histopathology was reported as primary mucinous adenocarcinoma of the urinary bladder invading the bladder muscularis propria, submucosa and urachus with perivesical fat margins being free of tumour [Table/Fig-2a]. Immunohistochemistry for CK7 [Table/Fig-2b], C20 [Table/Fig-2c] and BE12 [Table/Fig-2d] were 3+,2+ and 1+ respectively which confirmed primary bladder adenocarcinoma. The patient was subsequently referred to a medical oncologist and is currently doing well on surveillance follow up of three months.
[Table/Fig-1a] showing a contrast enhanced CT scan film depicting the bladder growth (arrow) in abutting in to the dome of the bladder. [Table/Fig-1b] depicting the excised enbloc specimen of partial cystectomy
[Table/Fig-2a] showing the H&E (10x) stained slide depicting histology of adenocarcinoma of urinary bladder, [Table/Fig-2b], [Table/Fig-2d] and [Table/Fig-2d] showing the immunohistochemistry of the specimen depicting cytokeratin CK7(3+strong), CK20 (2+strong) and B12(1+weak) positivivity confirming primary urachal bladder adenocarcinoma
DISCUSSION
Primary adenocarcinomas of the urinary bladder usually arise in middle aged men from the base of the bladder (50-60%) and or are associated with urachal remnants [1]. It is believed that majority of primary vesical adenocarcinoma may arise due to intestinal metaplasia, exsystrophy and persistent urachal remnants and while some may result from direct extension from adjacent organs (e.g.,colon, prostate) [2] and rarely due to metastatic spread to bladder from distant organ ( stomach or colon) [3]. Hematuria is the most common presenting symptom in about 90% of patients.
Urinary bladder adenocarcinoma, regardless of site, include the following histological variants:i) Adenocarcinoma (non-specific), ii) Adenocarcinoma of enteric type, iii) Adenocarcinoma with signet-ring cells, iv) Mucinous adenocarcinoma, v) Hepatoid adenocarcinoma, vi) clear cell carcinoma [4]. Mucinous adenocarcinoma of the urinary bladder includes extracellular mucin mixed with tumor cells. In some cases there is mixture of extracellular and intracellular mucin resulting in signet- ring configuration [5].
Prognosis varies with stage, with survival approaching 75-100% among patients whose tumors are confined to the urinary bladder [6]. Patients with urachal tumors tend to have better short-term survival rate than those with non-urachal cancers [7]. Immunoprofile of primary bladder and SRCC arising from the gastrointestinal tract overlaps, including positivity for CK7, CK20, CEA, EMA, CDX2, villin and E-cadherin. CK7 & CK20 are positive in contrast with colonic adenocarcinoma that express CK20 only.
At the time of diagnosis, about 25% of patients have distant metastases [8, 9], and approximately 50% have Stage IV disease (i.e., including node positivity, T4b primary tumour, as well as distant metastases)[8]. Prognosis of bladder adenocarcinoma is poor due to high tumour grade and the late presentation. Patients who receive no adjuvant therapy have an average survival of 3.5 onths [10] with an overall 1-year reported survival rate of 60% [9] and five year reported survival rate of 75%, 38% and 12% respectively for stage II, III and IV tumours [11]. Another recent study [8] reported 5-years survival rate of 50% for stages I-III, with no stage IV patients surviving beyond two years.
Partial cystectomy or transurethral resection may be indicated for small, well-demarcated primary bladder or urachal adenocarcinoma with long-term survival [12,13,14]. Radical cystectomy is generally favoured over partial cystectomy, particularly for non-urachal tumours, because of the possibility of local invasion being undetected on imaging [13]. In a single site study reviewing 21 cases of primary vesical adenocarcinoma the authors concluded that radical cystectomy may be more crucial than chemotherapy with survival strongly correlating with the stage of disease [15]. Adjuvant chemotherapy (5FU, methotrexate, cisplatin, mitomycin, doxocycline, tegafur, cyclophosphamide and carboplatin) [8] has been used in limited studies [7,8,9] with variable efficacy. Patients with advanced/metastatic disease who undergo chemotherapy and or radiation may improve disease free survival [1,8]. According to a recent clinical guide for treatment of urachal vesical adenocarcinoma the authors opined that due to a higher risk of post operative relapse reported especially in those with positive tumour margins, lymph node positivity, peritoneal involvement, or if an en-bloc resection of umbilicus was omitted predict a group of patients where one could consider adjuvant chemotherapy [16].
CONCLUSION
Primary adenocarcinoma of urinary bladder is an uncommon malignancy which may not be easy to differentiate from adenocarcinoma arising from the colon and prostate without immuno-histochemistry. We advocate early partial/radical cystectomy for localised bladder adenocarcinoma.
KEY MESSAGES
The present case highlights the diagnostic difficulties which are encountered in the establishment of a clinical and a histological diagnosis in a case of primary bladder adenocarcinoma.
[1]. Zaghoul S, Zaghloul MS, Aziz SA, Nouh A, Mohran TZ, Shazely S, Saber A, Primary Adenocarcinoma of the Urinary Bladder: Risk Factors and Value of Postoperative RadiotherapyJournal of Egyptian Nat. Cancer Inst 2003 15(3):193-200. [Google Scholar]
[2]. Torenbeek R, Primary signet-ring cell carcinoma of the urinary bladderHistopathology 1996 28:33-40. [Google Scholar]
[3]. Fiter L, Gimeno F, Martin L, Gomez Tejeda L, Signet-ring cell adenocarcinoma of bladderUrology 1993 41:30-33. [Google Scholar]
[4]. Eble JN, Epstein JI, Seternhenn IA, World Health Organization Classification of TumoursPathology and Genetics, Tumours of the Urinary System and Male Genital track 2004 128LyonIARC Press-32. [Google Scholar]
[5]. Marques ML, D’Alessandro GS, Chade DC, Lanzoni VP, Saiovici S, Ramos de Almeida JR, Primary mucinous adenocarcinoma of the bladder with signet-ring cells: case reportSao Paulo Med J 2007 125:297-99. [Google Scholar]
[6]. Sigalas Konstantinos, Tyritzis Stavros I, Trigka Eleni, Katafigiotis Ioannis, Kavantzas Nikolaos, Stravodimos Konstantinos G, A male presenting with a primary mucinous bladder carcinoma: a case reportCases Journal 2010 3:49 [Google Scholar]
[7]. Stenhouse G, Mcrae D, Pollock AM, Urachal adenocarcinoma in situ with pseudomyxoma peritonei: a case reportJ Clin Pathol 2003 56:152-53. [Google Scholar]
[8]. Akamatsu S, Takahashi A, Ito M, Ogura K, Primary signet-ring cell carcinoma of the urinary bladderUrology 2010 75:615-18. [Google Scholar]
[9]. Romics I, Szekely E, Szendroi A, Signet-ring cell carcinoma arising from the urinary bladderCan J Urol 2008 15:4266-4268. [Google Scholar]
[10]. Erdogru T, Primary signet ring cell carcinoma of the urinary bladder. Review of the literature and report of two casesUrol Int 1995 55:34-37. [Google Scholar]
[11]. Fiter L, Gimeno F, Martin L, Gomez Tejeda L, Signet-ring cell adenocarcinoma of bladderUrology 1993 41:30-33. [Google Scholar]
[12]. Holmang S, Borghede G, Johansson SL, Primary signet ring cell carcinoma of the bladder: a report of 10 casesScand J Urol Nephrol 1997 31:145-48. [Google Scholar]
[13]. Wang HL, u DW, Yerian LM, Immunohistochemical distinction between primary adenocarcinoma of the bladder and secondary colorectal adenocarcinomaAm J Surg Pathol 2001 25(11):1380-87. [Google Scholar]
[14]. Singh J, Zherebitskiy V, Grynspan D, Czaykowski M, Metastatic signet ring cell adenocarcinoma of the bladder: responsive to treatment?Can Urol Assoc J 2012 6(1):e15-19. [Google Scholar]
[15]. Zhang H, Jiang H, Wu Z, Fang Z, Fan J, Ding Q, Primary adenocarcinoma of the urinary bladder: a single site analysis of 21 casesInt Urol Nephrol 2013 45(1):107-11. [Google Scholar]
[16]. Siefker-Radtke A, Urachal adenocarcinoma: a clinician’s guide for treatmentSemin Oncol 2012 39(5):619-24. [Google Scholar]