E-cadherin is a transmembrane glycoprotein that mediates a cell–cell adhesion in the epithelial tissues [1]. Complete loss of the E-cadherin expression occurs in most invasive lobular carcinomas and lobular carcinomas in situ, but not in invasive ductal cancers or ductal carcinomas in situ [2].
The classification of invasive breast carcinomas is traditionally based on the histologic phenotype of the tumour cells. Breast carcinomas are classified as ductal and lobular carcinomas, based on the distinct morphological features of the tumour cells, as are noted on the microscopic examination. The histological criteria for the diagnosis of ILC includes loosely dispersed strands of infiltrating tumour cells which are arranged in the form of a single file, lack of cohesion without the formation of tubules or papillae, and small cells with relatively little nuclear pleomorphism, while ductal carcinoma is characterized by the presence of tumour cells which are arranged in tubules, trabeculae and sheets, which exhibit variable nuclear pleomorphism. Although the generally accepted histological criteria serve in distinguishing the lobular from the ductal carcinomas of the breast, the differential diagnosis may present a challenge in poorly differentiated carcinomas, which shows equivocal histological features with a diffuse infiltrating pattern and in the pleomorphic variant of invasive lobular carcinoma [3]. Lobular carcinoma in situ (LCIS) may be mimicked by low-grade ductal carcinoma in situ (DCIS), with a solid growth pattern which involves the terminal ducts and lobules [4]. Sometimes, separate foci of DCIS and LCIS may be seen and these may pose a problem [5]. A related problem is presented by the lesions with a typical lobular cytology, that have large areas of the central comedo-type necrosis. Finally, an in situ carcinoma which involves lobules that are composed of large, pleomorphic, discohesive tumour cells and signet-ring cells, with or without necrosis (the so-called pleomorphic LCIS), also may be difficult to classify [6, 7].
It has been reported that ILC has characteristics that are different from IDC, such as an older age at onset, a larger tumour size, an increased propensity for multifocality and multicentricity, and a higher risk for bilateral breast cancer. Hence, the distinction of an invasive lobular carcinoma from an invasive ductal carcinoma is clinically important. LCIS is managed conservatively, while DCIS is managed surgically, with the goal of complete removal of the tumor [8]. It was shown that the E-cadherin gene mutations and loss of the wild-type allele through loss of heterozygosity, were the predominant mechanisms by which the E-cadherin protein expression was lost frequently in lobular carcinoma, thus indicating that E-cadherin acted as a classic tumour suppressor gene. Thus, there is emerging evidence that E-cadherin is associated specifically with the lobular phenotype of breast carcinoma, and that the E-cadherin inactivation might have a crucial role in the dispersed and discohesive growth patterns in ILC. The loss of EC is from the outset, ie, in the pre-invasive stage of lobular carcinoma in situ (LCIS).
MATERIALS AND METHODS
This study was conducted on 28 cases each of invasive lobular carcinoma and ductal carcinoma of the breast. Grade II IDC tumours were selected for comparison, to eliminate the wide variation that exits in this type of invasive breast carcinomas. The tissues were processed routinely and stained with haematoxylin and eosin (H and E). The E Cadherin immunohistochemical staining was done on 4 μm thick paraffin sections by using the standard peroxidase-antiperoxidase method. All the steps were carried out in a moist and humid chamber, so that the sections remained moist throughout the procedure. The primary antibody which was used was the monoclonal mouse antihuman antibody E-Cadherin, Clone NCH-38 (DAKO). The presence of the E-cadherin staining in the epithelial cells of the normal ducts and the acini served as the positive internal control in every case.
The immunoreactivity with E-Cadherin was scored as follows: A strong inter-membranous staining in most of the tumour cells was scored as 3+ and a moderate staining in >10 % of the cells was scored as 2+, while a weak staining in < 10 % cells was scored as 1+, and an absence of membrane staining was scored as 0. The statistical analysis was performed by using the Chi-square test.
AIMS AND OBJECTIVES
To assess the E-Cadherin expression in breast carcinomas with lobular and ductal morphologies and its diagnostic utility in differentiating between the invasive lobular and ductal carcinomas and their in-situ components.
OBSERVATIONS
The diagnoses of the 28 cases were made, based on the histopathological examination and on the morphological features of the dyscohesive malignant cells, which were bland nuclear features which were arranged in the trabeculae in an Indian file pattern. Morphologically, 21 cases of ILC were diagnosed as classic, 3 as tubulo-lobular, 1 as solid, 2 as pleomorphic and 1 case as a signet ring cell variant. The incidence of IDC and ILC with in-situ component and their E-cadherin staining is shown in [Table/Fig-1 & 2].
Shows total no of ILC and IDC and in-suit components.
| Histological Type | Number of cases |
|---|
| ILC Alone | 22 |
| ILC with LCIS | 4 |
| ILC with DCIS | 2 |
| Total | 28 |
| IDC Alone | 15 |
| IDC with DCIS | 12 |
| IDC with both DCIS and LCIS | 1 |
| Total | 28 |
Shows E-Cadherin staining in IDC, LIC and in-situ component.
| Histological type of breast cancer | E-Cadherin staining score |
|---|
| 3+ | 2+ | 1+ | 0 (negative) |
| ILC (n=28) | 0 | 1 | 4 | 23 |
| LCIS component in ILC (n=4) | 0 | 1 | 0 | 3 |
| DCIS component in ILC (n=2) | 2 | 0 | 0 | 0 |
| IDC | 24 | 2 | 2 | 0 |
| DCIS component in IDC (n=12) | 10 | 1 | 1 | 0 |
| DLCIS component in IDC (n=1) | 1 (DCIS) | - | - | 1 (LCIS) |
A strong membrane (3+) expression of E-cadherin was identified in the benign epithelium, and 24/28 cases of IDC (85.7%) of tumours histologically diagnosed as invasive ductal carcinoma (NOS-Grade II), with a p value of <0.001. The E-cadherin expression was seen throughout the tumour in 100% of the cells in 22/28 of the IDC, whereas a focal loss of the membrane staining which involved a proportion of the tumour cells was seen in 4/28 cases. 13/14 cases (92.8%) of DCIS showed a strong 3+ complete intermembranous staining, whereas 1 case each showed a 2+ staining. The situ components of the same tumour were very similar. In contrast to the ductal carcinomas, none of the ILC cases showed a strong 3+ staining. A majority of the ILC cases (23/28) showed absence of the intermembranous staining pattern (p value: 0.0071). 1 case of ILC that showed the 2+ staining was a pleomorphic variant. Intensity of E-Cadherin staining in IDC and ILC shown in [Table/Fig-3]. Photomicrographs of different variants of ILC and IDC shown in [Table/Fig-4,5,6,7,8,9& 10].
Shows intensity of E-Cadherin staining in invasive and in-situ ductal and labular carcinoma.
| E-Cadherin Score | Invasive breast cancer | In-situ component |
|---|
| IDC (n=28) | ILC | P value | DCIS | LCIS | P value |
| (n=28) | (n=14) | (n=5) |
| 3+ | 24 | 0 | <0.001 | 13 | 0 | 0.0018 |
| 2+ | 2 | 1 | <0.001 | 13 | 0 | 0.0018 |
| 1+ | 2 | 4 | NS | 0 | 0 | NS |
| 0 | 0 | 23 | <0.001 | 0 | 4 | 0.0003 |
a) ILC: Trabeculae of bland appearing malignant cells, H&E 200X.
b) Negative E-Cadherin expression among tumor cells. Benign ducts serve normal positive internal control.
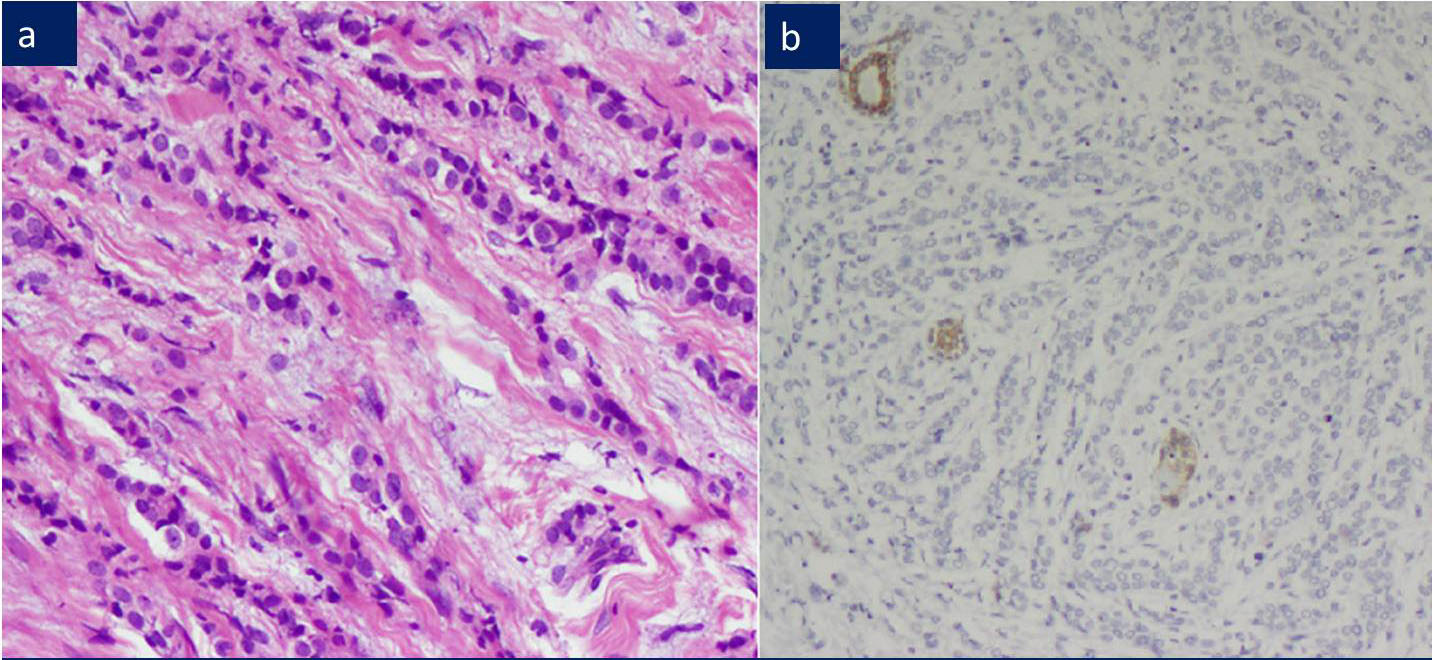
a) ILC-Tubulo-lobular variant, H&E 200X.
b) Negative E-Cadherin expression among tumor cells. Normal ducts stain positive.
a) ILC-Solid variant: Solid nests of bland appearing malignant cells, H&E 200X.
b) 1+ weak, incomplete intermembranous E-Cadherin expression among tomor cells.
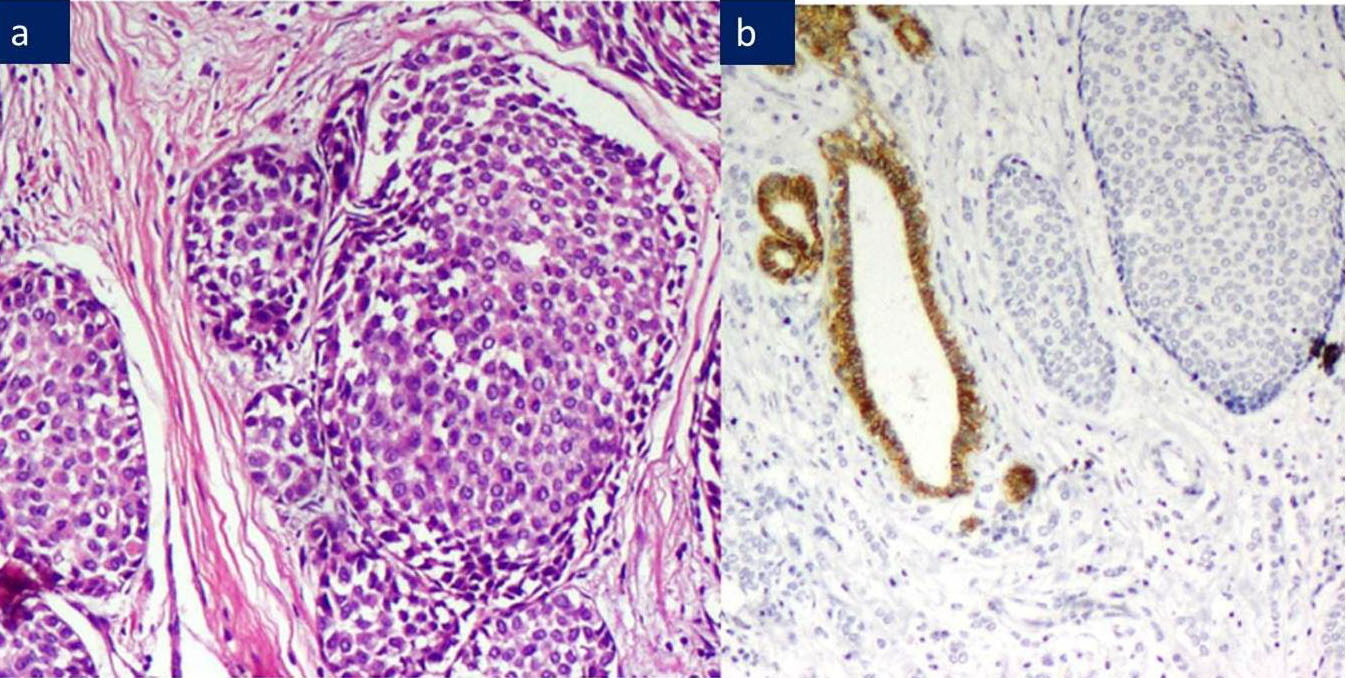
a) ILC: with foci of DCIS, H&E 200X.
b) ILC: Negative E-Cadherin, DCIS shows E-Cadherin 3+ positivity
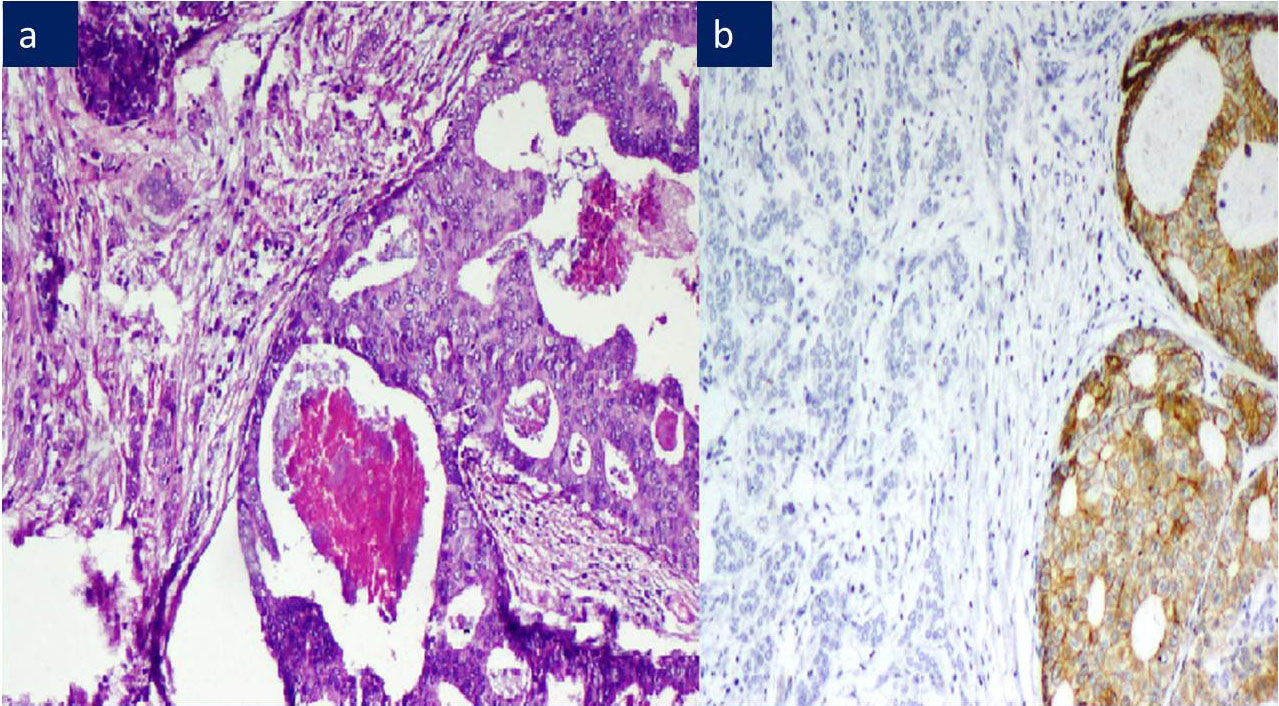
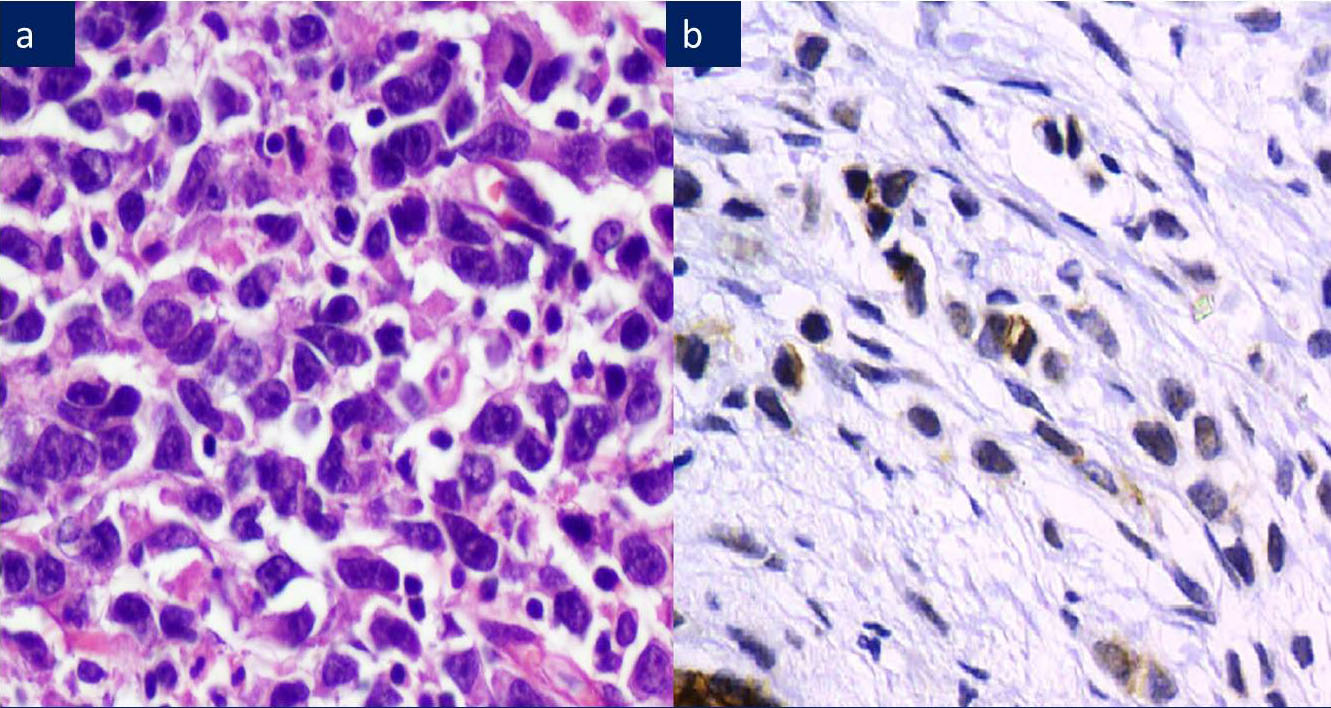
a) ILC: Signet ring cell type, H&E 200X.
b) ILC: 2+ membranous and cytoplasmic E-Cadherin positivity
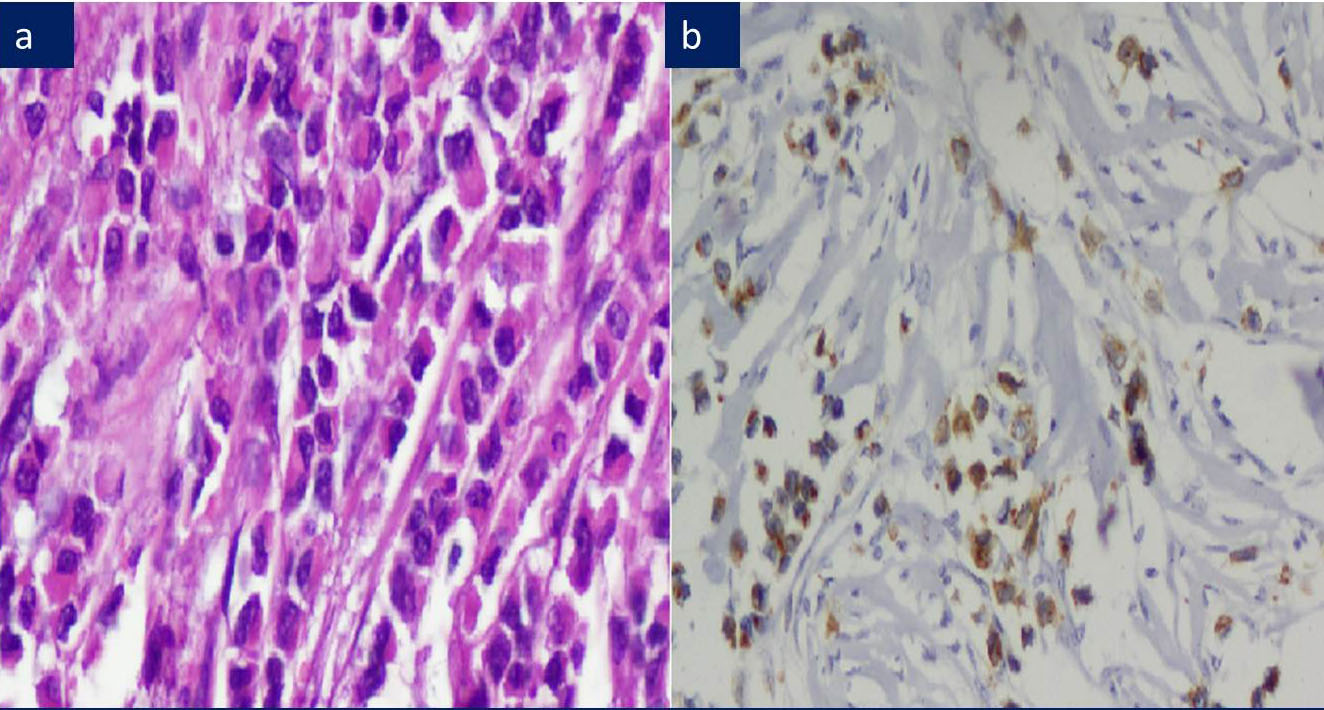
a) ILC: Pleomorphic type, H&E 200X.
b) ILC: Aberrant cytoplasmic & perinuclear dot E-Cadherin positivity
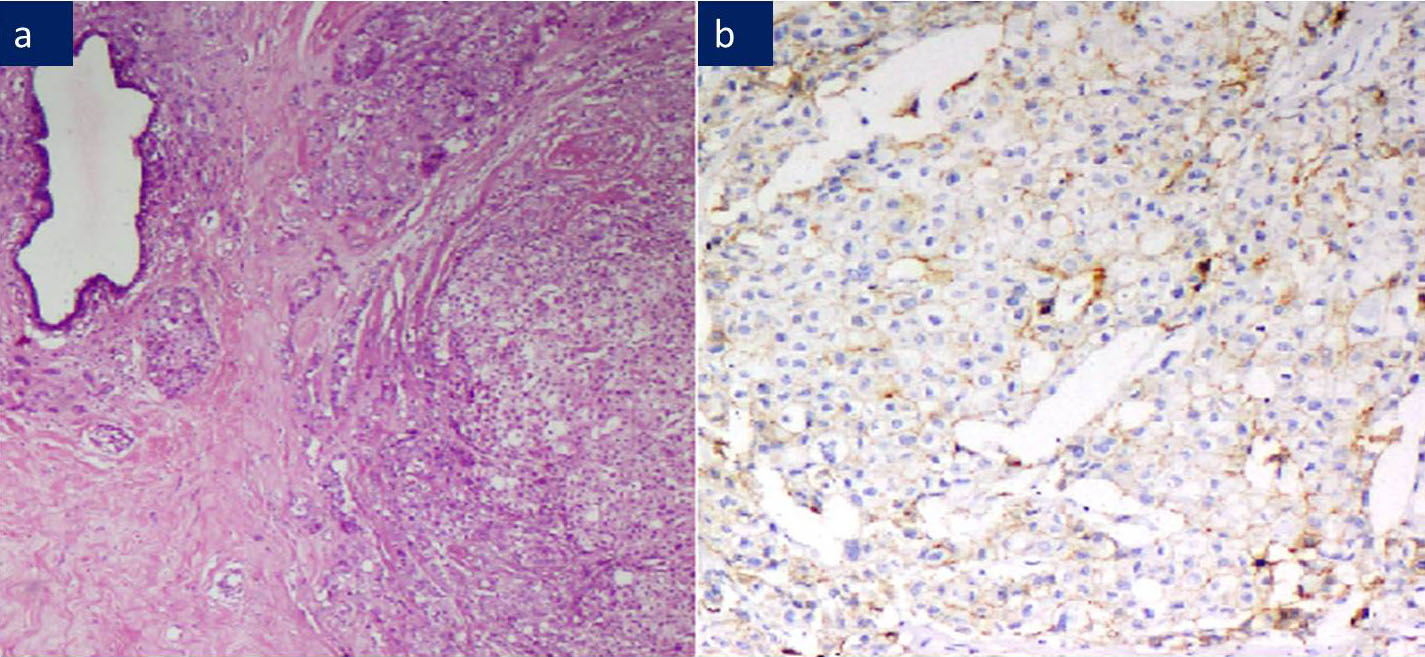
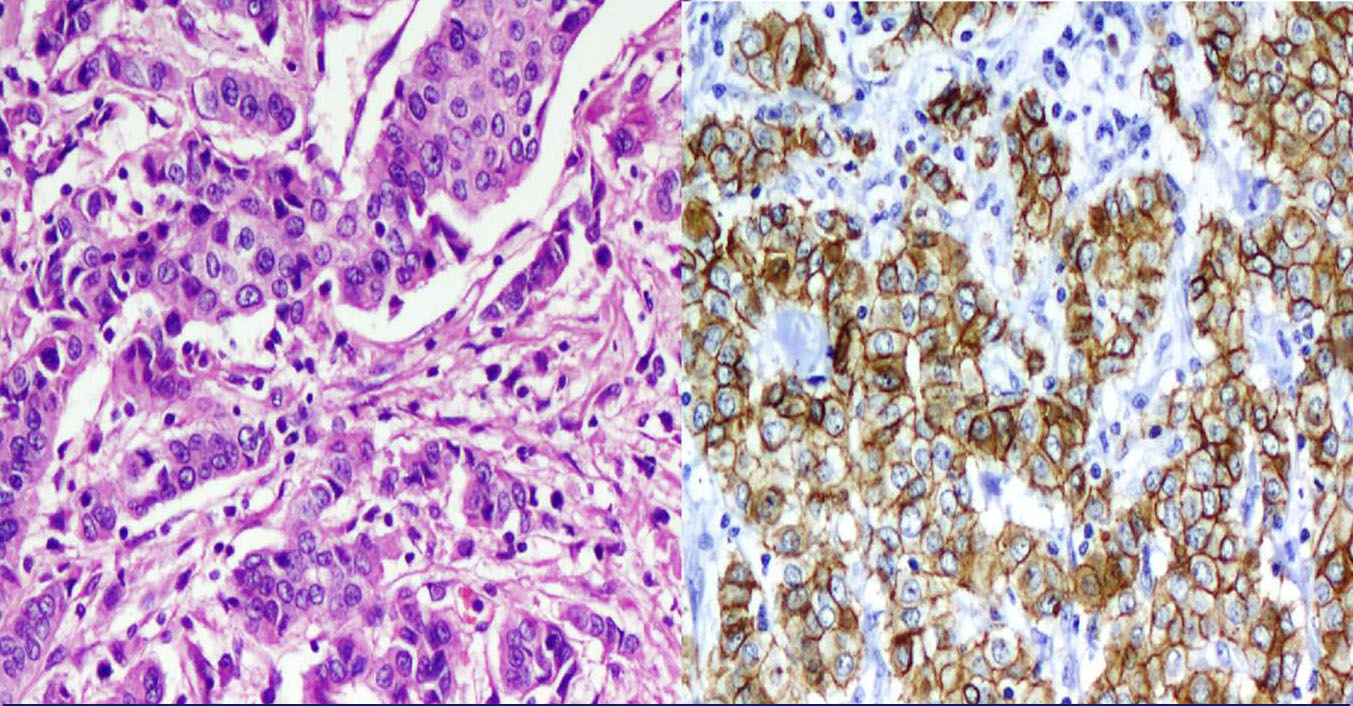
a) IDC: Malignant cells arranged in trabeculae and tubules, H&E 200X.
b) IDC: Majority IDC showed diffuse, complete, strong membranous E-Cadherin positivity
Four cases showed 1+ staining. Among 4 cases of LCIS, 3 showed complete loss, while 1 case showed 2+ staining. 1 case of IDC which showed separate foci of DCIS and LCIS, showed a marked difference in the staining of E-cadherin. While DCIS showed a 842strong uniform membrane staining, ILC demonstrated a complete loss of the membrane expression. We did not encounter any case of mixed lobular ductal carcinoma in our study, because we were able to classify most of the lesions as ductal or lobular, based on the cytoarchitectural features. 3 cases of the classic type of ILC (17.8%) demonstrated an aberrant E-cadherin expression in the cytoplasm or perinuclear dot like positivity.
DISCUSSION
Foote and Stewart used the term ‘lobular carcinoma in situ’ for a special type of non-invasive carcinoma of the breast which was associated with a monotonous intralobular proliferation of the cells [9]. The concurrent invasive carcinoma with the absence of a tubule formation and a single file pattern was established as ILC. The subsequent identification of the solid, alveolar, tubulo-lobular and the pleomophic variants added difficulty to the existing problem of distinguishing the IDC of no special type, with the cord like or the trabecular patterns, from ILC and its variants. The histological identification of ductal and lobular carcinomas has proven to be essential for the evaluation of the patients’ prognosis and determination of the treatment [10].
Loss of the E-cadherin expression validates ILC as a distinct entity and it explains its histological appearance and distinctive growth patterns in the metastases [11]. Although EC is emerging as an excellent marker, there are reports which have stated that the immunohistochemical expression of E-cadherin per se in the ILC histologic phenotypic tumours, should not preclude its diagnosis [12].
In our study, we found a highly significant correlation of the E-cadherin expression with the histological phenotype of the tumours. 26 of the 28 cases of IDC showed a moderate to strong membrane (2+/3+) expression of E-cadherin, while only 1/28 cases of ILC showed a 2+ staining. All other cases of ILC were negative for the E-cadherin expression. With regards to the in-situ lesions, 13/14 DCIS showed a 2+/3+ staining, while 1 of the 3 cases of LCIS showed a 2+ intensity. 1 case of DLCIS showed a strong expression in the DCIS component and a negative staining in the LCIS component. 1 case of ILC was diagnosed histologically as a pleomorphic lobular carcinoma. It showed a 2+ E-cadherin positivity. Given the well known difficulty in differentiating the pleomorphic variant from the invasive ductal carcinoma with a dispersed infiltrating pattern, this tumour most likely represents an example of the latter.
In a study which was done by Wiljo et al., an analysis of the E-cadherin expression which was done in 48 invasive ductal carcinomas and 38 invasive lobular carcinomas, showed that all the 48 invasive ductal carcinomas had the E-cadherin expression located on the plasma membrane, while 32 of the 38 invasive lobular carcinomas (84%) showed a complete loss of the E-cadherin expression [13]. In a study which was done by Gamallo et al and Moll et al., there was a complete loss of the E-cadherin expression in a majority of the ILCs, while all the cases of IDC retained some expression of E-cadherin [2, 14]. Our study results were comparable with those of the above studies.
A recent study which was done on a large series of cases of breast cancer showed a significant statistical correlation of the E-cadherin loss with a positive diagnosis of invasive lobular carcinoma and a negative E-cadherin stain confirmed the diagnosis of invasive lobular carcinoma with 97.7% specificity; 96.8% negative predictive value; 88.1% sensitivity; and 91.2% positive predictive value [15]. Some studies that showed a reduced expression of E-cadherin in IDC, were associated with a poor differentiation and high tumour grades [2, 14].
Diagnostic difficulties occur in some cases, because IDC may show a dispersed growth pattern, which includes infiltration around the benign ducts in a targetoid manner, which is similar to that in ILC. A positive E-cadherin expression, in such cases, may help in classifying the lesions as IDC with a dispersed growth pattern. 9 out of 276 cases of invasive breast cancers which were initially diagnosed as invasive cancers of an uncertain type, were re-classified into ILC and IDC, based on the E-Cadherin staining, in a study which was done by R Singhai et al., [15].
The identification of the solid, alveolar, tubulo-lobular, and the pleomorphic variants of ILC has added new dilemmas to the existing problem of distinguishing the IDC from the ILC variants [16, 17]. Studies have shown that the loss of E-Cadherin can be reliably checked, for distinguishing the ILC variants from IDC. In our study, the variants of ILC, like the tubulo-lobular and the solid variants, showed loss of the E-Cadherin expression.
Interestingly, we noted that 5 cases of ILC showed an aberrant expression of E-cadherin, with 3 cases showing a cytoplasmic expression, and 2 of them showing a perinuclear dot like positivity. Such an expression was also reported by Moll et al., [10]. This is thought to occur because of a mutant E-cadherin which is incorrectly processed within the Golgi apparatus. An aberrant expression of E-Cadherin, in the form of a weak, dot-like cytoplasm or a discontinuous membrane reaction, was seen in 15.5% of the breast cancers of both the lobular- or the ductal-types, in a study which was done by Choi YJ et al., [18]. An aberrant E-cadherin reaction was observed in previous studies, in the range of 0.4–54%. Goldstein et al., reported 9% lobular carcinoma in situ cases with the E-cadherin membrane expression, which was seen usually as a patchy distribution and of a lesser intensity than was noted in duct carcinoma in situ [19].
Misinterpretation of the “aberrant” positive staining may lead some to exclude a diagnosis of lobular carcinoma. An erroneous classification of the tumours may lead to a mismanagement of the patients in the clinical practice, particularly in the context of the in situ disease at the margins.
Da Silva et al., in their study, noted that 4/25 ILCs had some positive staining for E-cadherin and tha the “aberrant positivity” was observed as incomplete membrane, cytoplasmic, and/or Golgi type staining patterns [20]. However, they suggested that the E-cadherin protein may be nonfunctional in these cases, as studies showed β-catenin to be completely negative in 2 cases, which demonstrated a failure of the cadherin-catenin complex formation that was required for normal cell functions and maintenance of the tissue architecture, which included cell-cell adhesion. In the remaining 2 cases, the cells which exhibited a complete and linear membrane staining for both E-cadherin and/or β-catenin were admixed with the cells with an “aberrant positivity”, which was observed as incomplete membrane, cytoplasmic, and/or Golgi type staining patterns. Again this suggested a failed cadherin-catenin complex formation. Hence, they suggested that the E-cadherin positivity was not an excluded event in the diagnosis of lobular carcinoma.
CK [8] and the high molecular weight cytokeratin have been used to distinguish between the ductal and the lobular lesions [21]. However, we did not use these antibody markers in our study.
CONCLUSION
There was a positive correlation between the E-cadherin expression and IDC and the loss of E-cadherin and the diagnosis of ILC and its variants.
Currently, the classification of breast carcinoma is purely done, based on the histological examination. Although the well accepted histological features served in classifying and enhancing our ability in classifying breast carcinomas into invasive ductal and lobular carcinomas, in ambiguous cases with histologically equivocal features, a strong, complete, membranous E-cadherin expression may help in resolving the problem and in aiding in the subclassification of invasive breast carcinoma. However, an aberrant cytoplasmic/ perinuclear expression of E-cadherin should not be considered as a positivity.