Alveolar Soft Part Sarcoma-A Histological Surprise in a Male Patient who was Suspected to have Breast Cancer
Sunitha Susan Varghese1, Balukrishna Sasidharan2, Subramaniam Kandasamy3, Marie Therese Manipadam4, Selvamani Backianathan5
1 Assistant Professor, Department of Radiation Oncology, Unit 1, CMC, Vellore, Tamil nadu, India.
2 Assistant Professor, Department of Radiation Oncology, Unit 1, CMC, Vellore, Tamil nadu, India.
3 Assistant Professor, Department of Pathology, CMCVellore, Tamil nadu, India.
4 Professor, Department of Pathology, CMCVellore, Tamil nadu, India.
5 Professor, Department of Radiation Oncology, Unit I, CMCVellore, Christian Medical College, Tamil Nadu, India.
NAME, ADRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Sunitha Susan Varghese, Assistant Professor, Department of Radiation Oncology, Unit I, CMC Vellore, Tamil nadu, 632004, India.
Phone: 91-9943062385
E-mail: sunithasusan@cmcvellore.ac.in
Alveolar Soft Part Sarcoma (ASPS) is a very rare type of soft tissue sarcoma. Its cell of origin is unclear. It usually presents in the second to fourth decade of life. The most common reported sites of ASPS are the lower extremities, the head and the neck. Because of the rarity of this disease, there is no standard treatment plan. Surgical excision with negative margins is considered as the treatment of choice. We are reporting a rare presentation of ASPS as a male breast lump.
ASPS, Sarcoma, Male, Breast lump
INTRODUCTION
ASPS forms less than 1% of all the soft tissue sarcomas [1]. Its cell of origin is unclear. Being a rare tumour, not much information is available on its clinical features and on the appropriate treatment. ASPS most commonly presents in the lower extremities [1,2]. ASPS has distinctive histologic features, but it can be deceptive when it arises in unusual locations. Previously, two cases of ASPS which arose in the breast had been reported [2]. Here, we are reporting a case of ASPS which arose in the male breast, which was suspected to be male breast cancer, but which turned out to be ASPS after a histological examination.
CASE REPORT
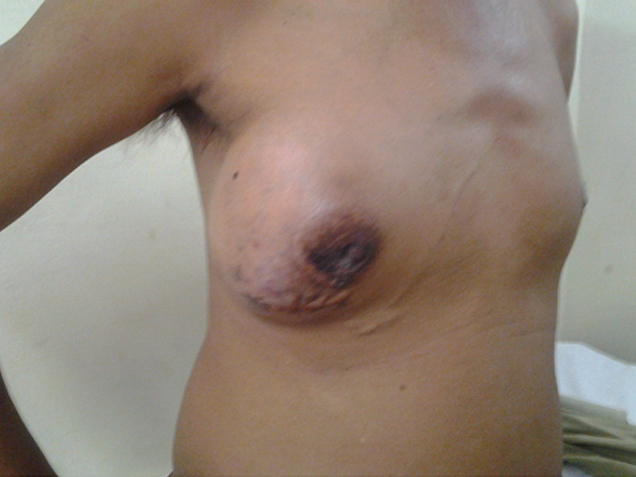
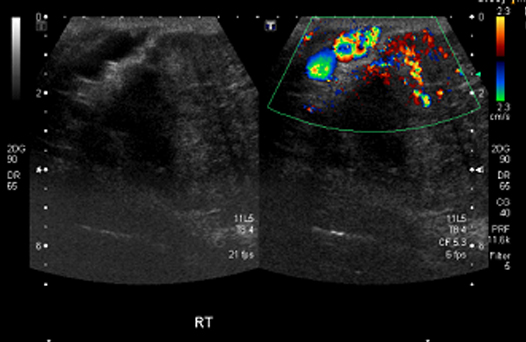
A forty six years old business person presented with a swelling in his right breast, which was of seven years duration, which was slowly progressive in nature. There was no associated pain or nipple discharge. He gave a history of an excision of a similar swelling in the same breast, which was done 20 years ago. He had no symptoms for thirteen years, after which the lump had recurred. On clinical examination, it was found that there was a 15 x 10 cm swelling in his right breast [Table/Fig-1], which was fixed to the pectoralis major muscle but which was not fixed to the chest wall. There were dilated veins in the skin which overlay the lump. There were no palpable axillary lymph nodes. An ultrasonography which was done, revealed a vascular tumour [Table/Fig-2]. A tru-cut biopsy from the lump was reported as a granular cell tumour. However, due to the lymphovascular emboli which were seen in the biopsy specimen, the possibility of a malignancy was considered. His metastatic work up was negative. He underwent a right simple mastectomy and an axillary dissection.
Clinical picture showing lump in a male right breast. Dilated veins seen on the overlying skin
Ultrasonogram of the breast lump showing increased vascularity
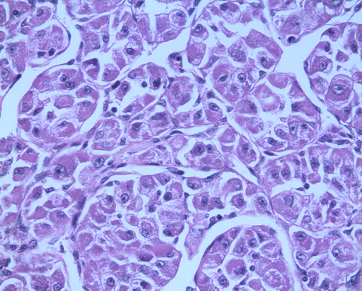
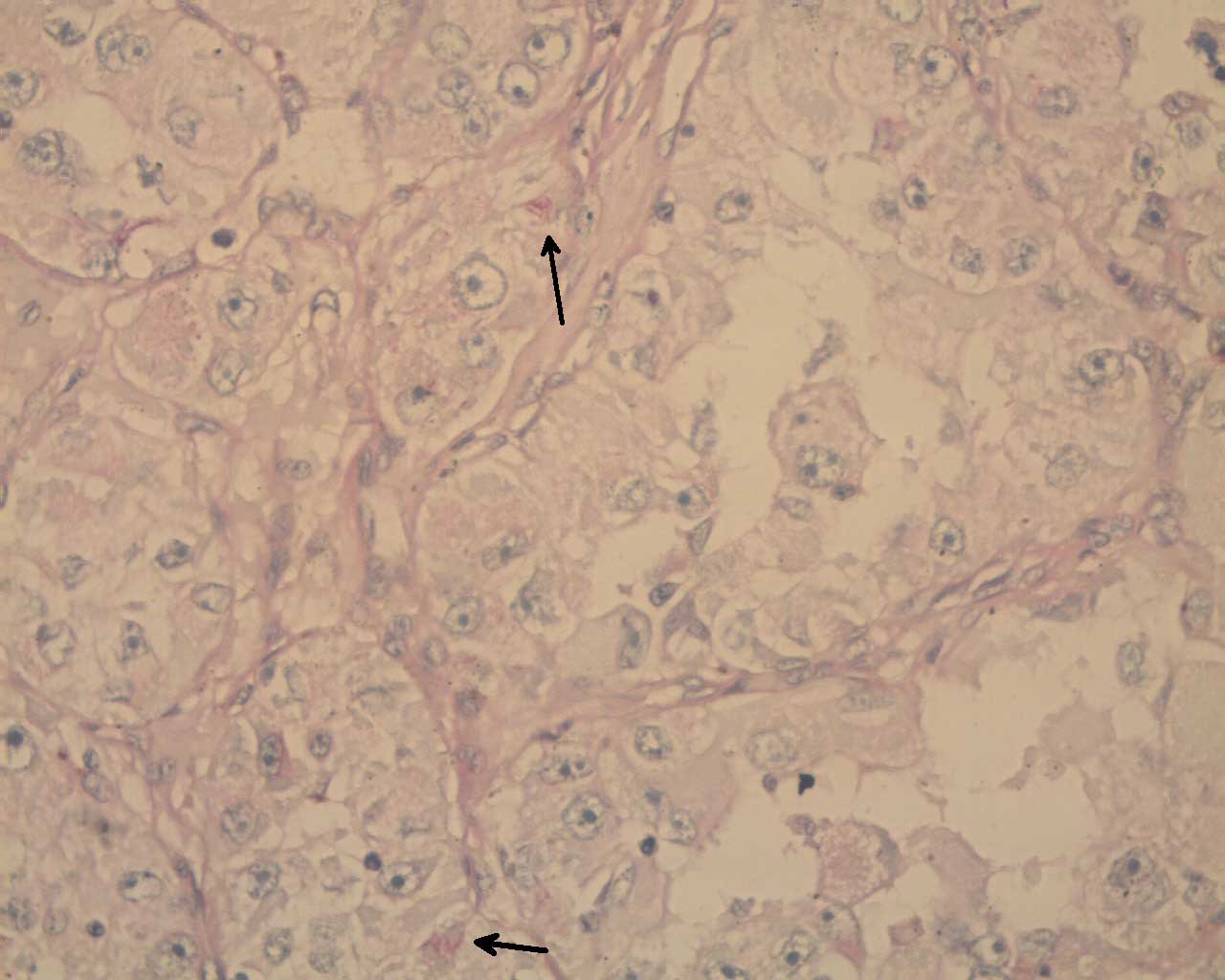
The surgical pathology specimen report was that of an alveolar soft part sarcoma with a maximum tumour size of 11.5 cm. The tumour cells were arranged in nests, with loss of central cohesion. The nests were separated by thin fibro vascular septae [Table/Fig-3]. The cells had abundant eosinophilic cytoplasm with large nuclei. They had prominent nucleoli. These cells contained PAS positive and diastase negative cytoplasmic structures [Table/Fig-4]. There was lymphovascular invasion. Ten axillary lymph nodes were negative for metastases. All the margins were negative, but the deep resection margin was close (< 1 mm). The immunohistochemistry showed that the tumour cells were positive for S-100, Desmin and CD-68 and negative for cytokeratin. The neuron specific enolase was non-contributory. The MIB 1 index at the area of active proliferation was 10%.
H & E, 400X, showing alveolar pattern of cellular arrangement in ASPS
PASD, 400X, arrows showing PAS positive, diastase resistant cytoplasmic structures in ASPS cells
He received radiation therapy to his right chest wall with the use of the Medial and lateral tangent technique, in which 54 Gy in 27 fractions were delivered. He tolerated his treatment well. He is on regular follow up and was disease free at the time of the last follow up, at eight months.
DISCUSSION
Christopherson et al initially described ASPS in 1952 [3]. He coined the term ‘alveolar soft part sarcoma’ due to its characteristic histological pattern, which gives a pseudo alveolar appearance. Making an accurate diagnosis of ASPS requires a high index of clinical suspicion and a clinicopathological correlation.
ASPS often presents as a soft, painless, slow growing tumour. The most common location is the lower extremity [1]. ASPS has been reported in other sites such as the mediastinum, the Gastrointestinal Tract (GIT), the bone, the trunk, the retro peritoneum, and in the head and neck sites [4–6]. ASPS has high rates of metastases. The most common metastatic sites are the lung, brain, bone and 750the liver [6]. It has been reported that ASPS has a high incidence of brain metastases (about 30%), almost three times higher than other soft tissue sarcomas [1,7].
These tumors are vascular, which is evident on a radiological evaluation. Sometimes, there can be areas of necrosis within the tumour. MRI scans show hyper intense signals in the T1 and T2 weighted images [8].
ASPS has a characteristic light microscopic appearance. The ASPS cells have well defined borders with abundant eosinophilic to clear cytoplasm [9]. Occasionally, the cytoplasm can be granular. They have round eccentrically placed nuclei with prominent nucleoli. Their mitotic activity is low. The cells are arranged in nests, which are separated from each other by thin fibrous septa. There are abundant vascular channels within the septae.
Shipkey et al., [10] detailed the ultra structural features of ASPS in 1964. Ultrastructurally, the tumour cells have numerous mitochondria and a smooth endoplasmic reticulum [9]. There are extensive Golgi body complexes with the multiple granules which are within and around it [9]. ASPS is characterized by the presence of intracytoplasmic rhomboid shaped crystalline structures which stain with PAS and which are resistant to diastase [9].
Immunohistochemistry has an important role in the diagnosis of ASPS. The ASPS cells do not stain with the antibodies which are against the epithelial markers such as cytokeratin and the epithelial membrane antigen [9,11,12]. They stain negative for the specific neuroendocrine markers like chromogranin A and synaptophysin [9,11,12]. They also stain negative for the specific melanocyte markers like HMB 45 and melanin A [9,11,12]. Sometimes, the tumour cells may stain positive for the S100 protein and neuron specific enolase. The ASPS cells may stain positive for the myogenic markers like Actin and Desmin. They may also stain positive for Vimentin and the Z protein [9,11,12]. The ASPS cells express Myo D1, which is a marker for skeletal muscle differentiation [9]. ASPS is characterized cytogenetically by a translocation between chromosome X and chromosome 17. This causes the fusion of the TEF3 gene on Xp11 to the ASPL gene on 17q25 [13]. The consistent detection of the ASPL - TFE3 fusion in ASPS, identifies it as a molecular diagnostic marker for ASPS [13]. The differential diagnosis of ASPS includes paraganglioma, granular cell tumour, renal cell carcinoma and malignant melanoma [12].
The incidence of distant metastases in ASPS is very high, but the 5 year overall survival ranges from 50 – 88% [1]. The tumour size and the stage were significant prognostic factors for the overall survival [1]. Metastatectomy is an option for those patients with a good performance status and for those tumours that can be completely resected with acceptable morbidity. The presentation as a metastatic disease decreases the survival rates. Surgical excision of the primary tumour with microscopically negative margins is the main stay of the treatment for ASPS [1]. Achievement of complete microscopic resection is critical in localized alveolar sarcoma [14]. The local recurrence after the excision of ASPS can be as high as 20% [5]. Adjuvant radiation therapy is offered when there is an increased chance of a local recurrence like close or positive margins [5,15]. Sherman et al., [15] reported that radiation therapy had prolonged local control in patients with ASPS. Radiation therapy was also found to be useful in slowing the growth of the metastatic deposits and for providing a meaningful palliation.
Chemotherapy in various combinations of antineoplastic drugs (Doxorubicin/Ifosfomide/Cyclophosphomide/Cisplatin) is offered for metastatic disease [5]. Various chemotherapeutic regimens have shown only a limited benefit in the treatment of ASPS [7]. Hence, chemotherapy as an adjuvant treatment, is not recommended. There are anecdotal reports on the tumour response in ASPS to interferon alpha [16].A targeted therapy with antiangiogenic agents like bevacizumab and sunitinib may also have a role in the treatment of ASPS [17,18].
CONCLUSION
Alveolar soft part sarcoma is a slow growing tumour and it has a high incidence of distant metastases, possibly due to its high vascularity. Its presentation in unusual locations can be deceptive. An early diagnosis and excision of the tumour with negative margins, is important in the treatment of ASPS. Radiotherapy has a role in the treatment of ASPS with close or positive margins. The role of chemotherapy in ASPS is not well defined. Antiangiogenic agents have shown a reasonable benefit in the treatment of ASPS.
[1]. Ogura K, Beppu Y, Chuman H, Yoshida A, Yamamoto N, Sumi M, Alveolar soft part sarcoma: a single-center 26-patient case series and review of the literatureSarcoma 2012 doi:10.1155/2012/907179 [Google Scholar]
[2]. Wu J, Brinker DA, Haas M, Montgomery EA, Argani P, Primary alveolar soft part sarcoma (ASPS) of the breast: report of a deceptive case with xanthomatous features confirmed by TFE3 immunohistochemistry and electron microscopyInt. J. Surg. Pathol 2005 Jan 13(1):81-85. [Google Scholar]
[3]. Christopherson WM, Foote FW, Stewart FW, Alveolar soft part sarcomas: structurally characteristic tumors of uncertain histogenesisCancer 1952 5(1):100-11. [Google Scholar]
[4]. Ordóñez NG, Alveolar soft part sarcoma: a review and updateAdv Anat Pathol 1999 May 6(3):125-39. [Google Scholar]
[5]. Lieberman PH, Brennan MF, Kimmel M, Erlandson RA, Garin-Chesa P, Flehinger BY, Alveolar soft-part sarcoma. A clinico-pathologic study of half a centuryCancer 1989 Jan 1 63(1):1-13. [Google Scholar]
[6]. Portera CA Jr, Ho V, Patel SR, Hunt KK, Feig BW, Respondek PM, Alveolar soft part sarcoma: clinical course and patterns of metastasis in 70 patients treated at a single institutionCancer 2001 Feb 1 91(3):585-91. [Google Scholar]
[7]. Reichardt P, Lindner T, Pink D, Thuss-Patience PC, Kretzschmar A, Dörken B, Chemotherapy in alveolar soft part sarcomas. What do we know?Eur. J. Cancer 2003 Jul 39(11):1511-16. [Google Scholar]
[8]. Temple HT, Scully SP, O’Keefe RJ, Rosenthal DI, Mankin HJ, Clinical presentation of alveolar soft-part sarcomaClin Orthop Relat Res 1994 Mar (300):213-18. [Google Scholar]
[9]. Folpe AL, Deyrup AT, Alveolar soft-part sarcoma: a review and updateJ. Clin. Pathol 2006 Nov 59(11):1127-32. [Google Scholar]
[10]. Shipkey FH, Lieberman PH, Foote FW Jr, Stewart FW, Ultrastructure of alveolar soft part sarcomaCancer 1964 Jul 17:821-30. [Google Scholar]
[11]. Persson S, Willems JS, Kindblom LG, Angervall L, Alveolar soft part sarcoma. An immunohistochemical, cytologic and electron-microscopic study and a quantitative DNA analysisVirchows Arch A Pathol. Anat Histopathol 1988 412(6):499-513. [Google Scholar]
[12]. Zarrin-Khameh N, Kaye KS, Alveolar soft part sarcomaArch. Pathol. Lab. Med 2007 Mar 131(3):488-91. [Google Scholar]
[13]. Ladanyi M, Lui MY, Antonescu CR, Krause-Boehm A, Meindl A, Argani P, The der(17)t(X;17)(p11;q25) of human alveolar soft part sarcoma fuses the TFE3 transcription factor gene to ASPL, a novel gene at 17q25Oncogene 2001 Jan 4 20(1):48-57. [Google Scholar]
[14]. Kayton ML, Meyers P, Wexler LH, Gerald WL, La Quaglia MP, Clinical presentation, treatment, and outcome of alveolar soft part sarcoma in children, adolescents, and young adultsJ Pediatr Surg 2006 Jan 41(1):187-93. [Google Scholar]
[15]. Sherman N, Vavilala M, Pollock R, Romsdahl M, Jaffe N, Radiation therapy for alveolar soft-part sarcomaMed. Pediatr Oncol 1994 22(6):380-83. [Google Scholar]
[16]. Roozendaal KJ, De Valk B, Ten Velden JJA, Van der Woude HJ, Kroon BBR, Alveolar soft-part sarcoma responding to interferon alpha-2bBr. J. Cancer 2003 Jul 21 89(2):243-45. [Google Scholar]
[17]. Vistica DT, Hollingshead M, Borgel SD, Kenney S, Stockwin LH, Raffeld M, Therapeutic vulnerability of an in vivo model of alveolar soft part sarcoma (ASPS) to antiangiogenic therapyJ Pediatr Hematol Oncol 2009 Aug 31(8):561-70. [Google Scholar]
[18]. Stacchiotti S, Tamborini E, Marrari A, Brich S, Rota SA, Orsenigo M, Response to sunitinib malate in advanced alveolar soft part sarcomaClin. Cancer Res 2009 Feb 1 15(3):1096-104. [Google Scholar]