Bullous Fixed Drug Eruption to Ciprofloxacin: A Case Report
Sonia Pramod Jain1, Pramod Ajit Jain2
1 Professor, Department of Skin and V.D., Mahatma Gandhi Institute of Medical Sciences, Sewagram, Wardha-442102, India.
2 Professor, Department of Orthopedics, Mahatma Gandhi Institute of Medical Sciences, Sewagram, Wardha-442102, India.
NAME, ADDRESS, E-MAIL ID OF THE CORESPONDING AUTHOR: Dr. Sonia Pramod Jain, Department of Skin & V.D., Mahatma Gandhi Institute of Medical Sciences, Sewagram, Wardha-442102, India.
Phone: 09370868133
E-mail: soniapjain@rediffmail.com
Adverse reactions to medications are extremely common and display a characteristic clinical morphology such as fixed drug eruption (FDE), Stevens-Johnson syndrome, urticaria, morbilliform exanthem, hypersensitivity syndrome, pigmentary changes, lichenoid, dermatitis, acute generalized exanthematous pustulosis, photosensitivity, vasculitis etc. Here we report a case of a 60 year old male who presented to us with multiple bullous eruptions over both the hands and feet after oral ingestion of ciprofloxacin.
Ciprofloxacin, Drug reaction
INTRODUCTION
The prevalence of drug eruptions has been reported in the range of 2-5% [1]. Fixed drug eruptions may account for as much as 16-21% of all cutaneous drug eruptions. Several variants of fixed drug eruption have been described, based on their clinical features and the distribution of the lesions [2,3,4,5,6,7] like generalized or multiple,linear,bullous,urticarial, pigmenting, nonpigmenting, wandering, eczematous, psoriasiform, erythema dyschromicum perstans–like, vulvitis and oral fixed drug eruption. Here we describe a case of bullous fixed drug eruption to ciprofloxacin which is a widely used quinolone antibiotic. The drug is known to cause cutaneous adverse reactions in 1% to 2% of the treated patients [8]. Fluoroquinolones commonly cause a morbilliform rash and/or photosensitivity, but rarely result in FDE [9]. The few cases of fluoroquinolone-induced FDE reported in the literature are typically localized FDE. A case of a generalized FDE following treatment with ciprofloxacin and levofloxacin has also been reported [10]. Manifestations most commonly attributed to the drug are urticaria, angioedema, maculopapular exanthem and photosensitivity [11]. However, there have been reports of a few cases in which ciprofloxacin has been implicated in fixed drug eruption, Steven Johnson Syndrome or toxic epidermal necrolysis. Here we describe a case of bullous variety of fixed drug eruption induced by ciprofloxacin as this type of presentation due to any drug is in itself very rare.
CASE SUMMARY
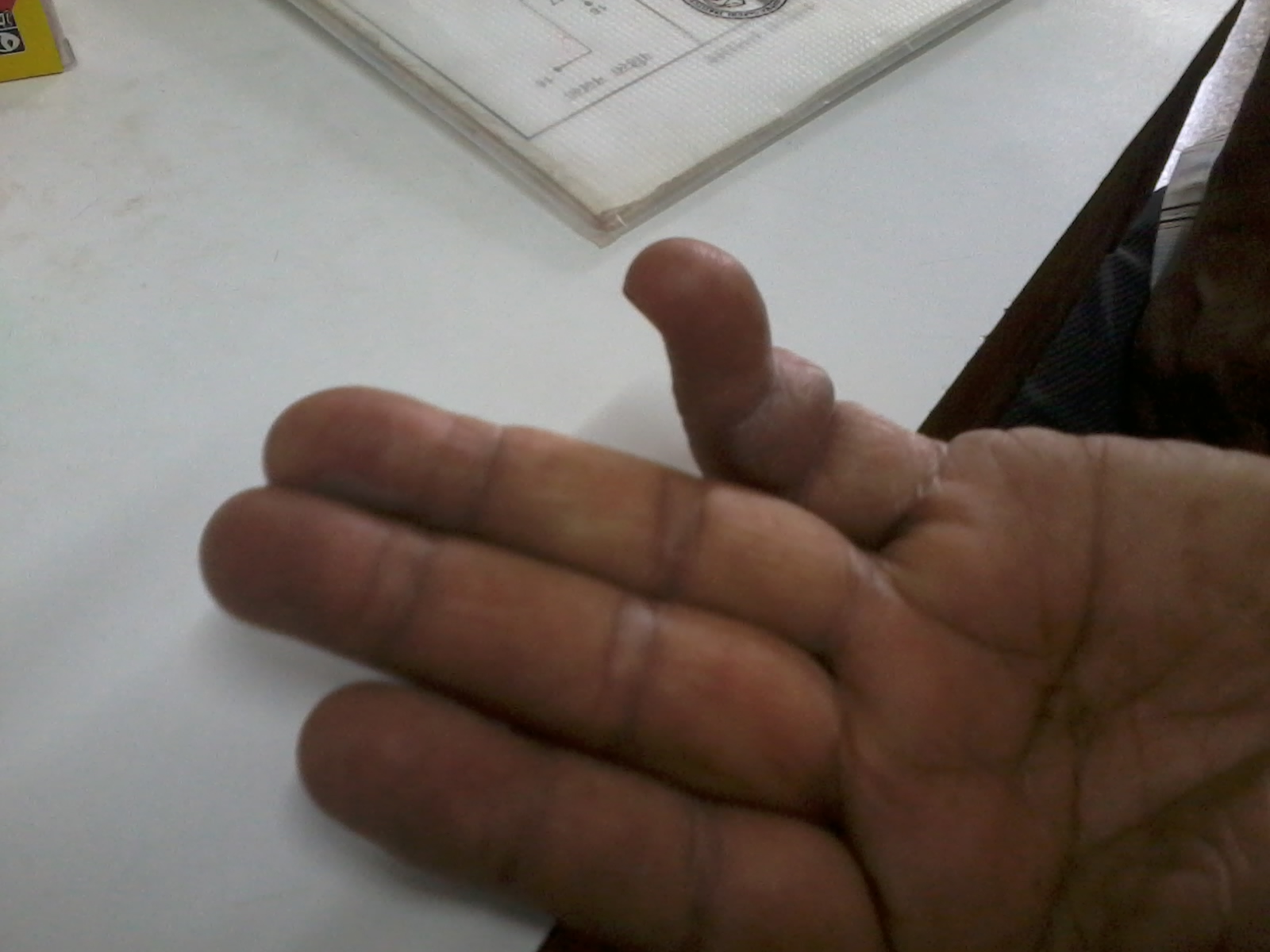
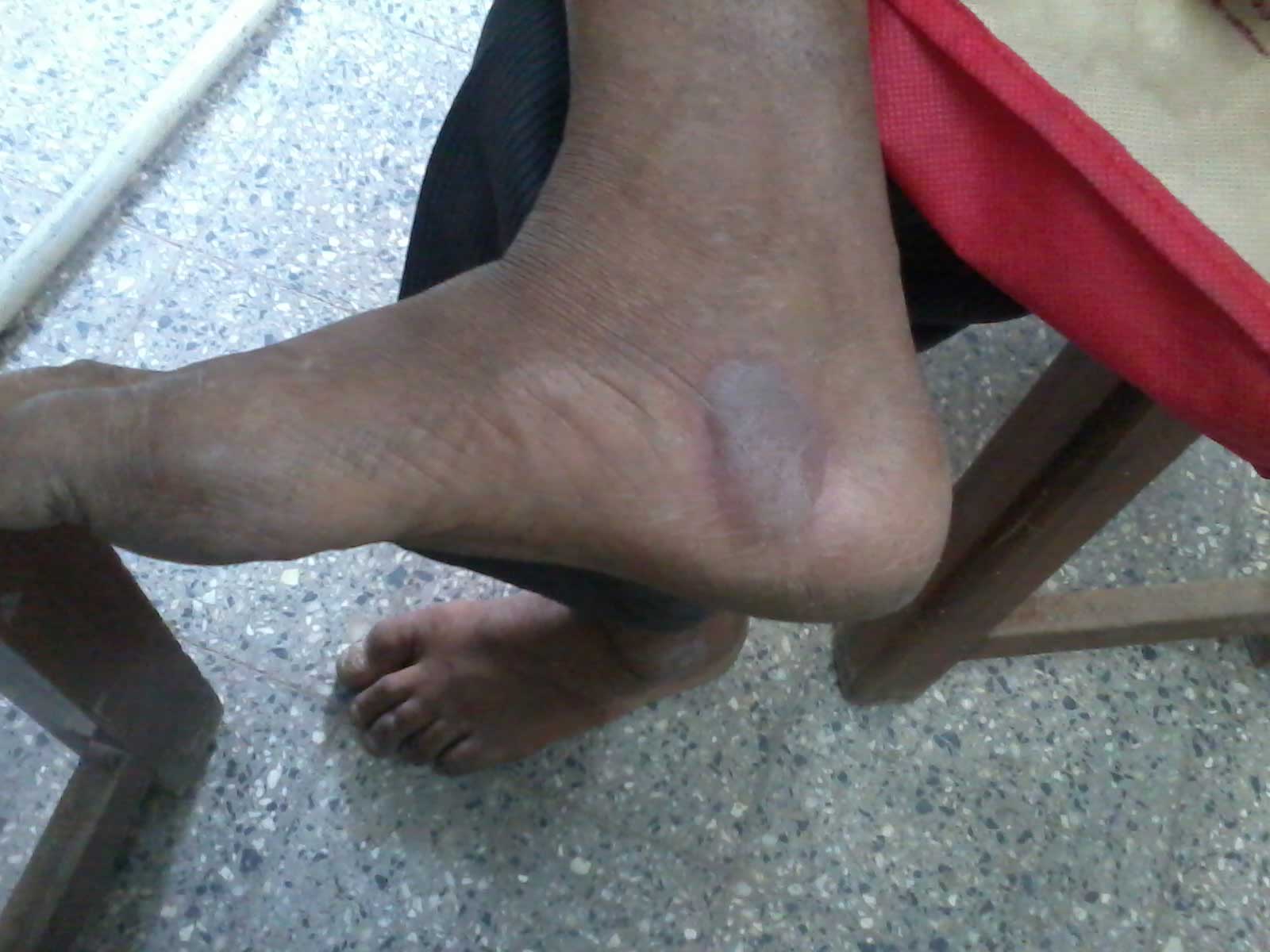
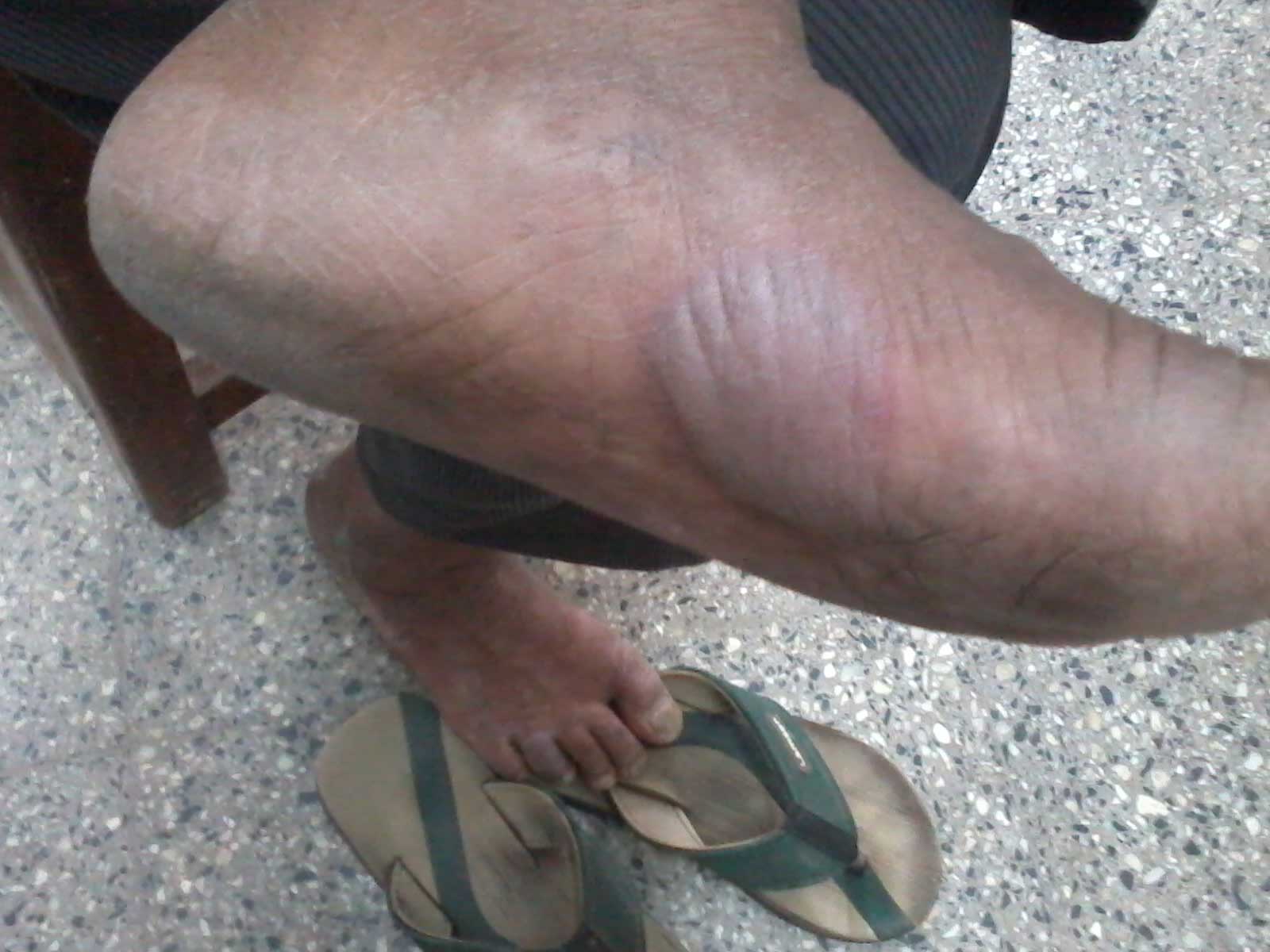
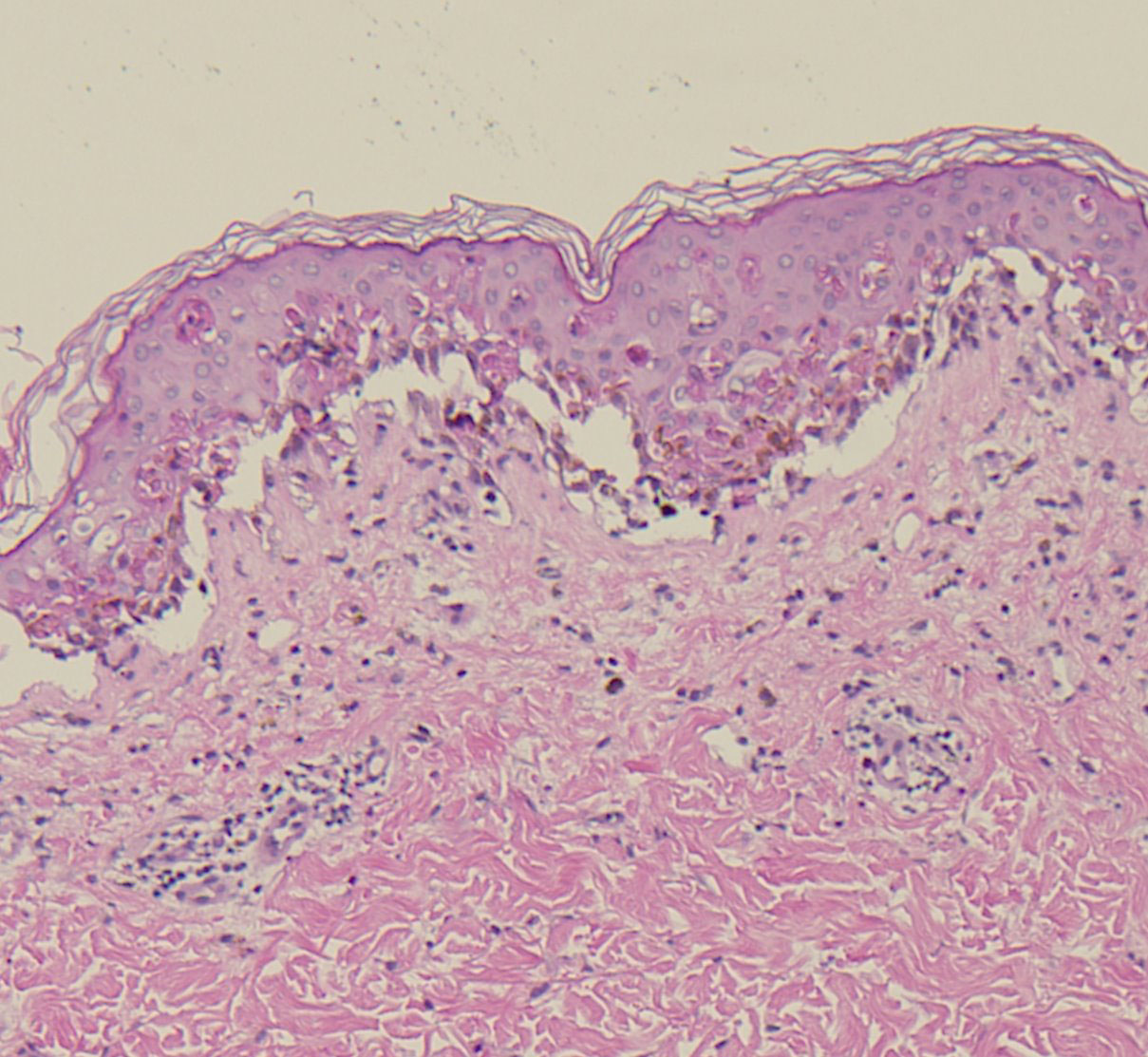
A 60 year old male presented to us with 2 day history of multiple fluid filled purplish blisters over both the hands and feet [Table/Fig-1,2 &3]. He stated that the lesions appeared within a few hours of taking a single dose of oral ciprofloxacin which was prescribed to him post operatively after his cataract surgery by a private practioner. Also, he had been using ciprofloxacin eye drops for the same. He began itching over both the hands and feet followed by burning sensation and the subsequent development of multiple fluid filled purplish lesions. He also developed erosions over the lips. He had no significant past medical or surgical history. There was no history of any other oral drug intake. His physical examination revealed multiple purplish-livid bullae over the fingers of both the hands and over the feet. Erosions with crusting were evident over his lips. There was congestion and swelling over his left eye, the one operated for cataract. He had no genital lesions though he complained of itching over the genitals. He was afebrile and his vitals were within the normal range. He was treated with oral corticosteroids with an advice to strictly avoid the drug in future. The diagnosis of bullous fixed drug eruption was made on clinical and histopathogical grounds along with the fact that he had past history of a similar but milder reaction to the drug 6 months back which he had taken for upper respiratory tract infection. However, oral provocation test and patch testing with ciprofloxacin could not be done as the patient did not consent for the same. The histopatholgical findings were consistent with those of fixed drug eruption with interface dermatitis along with vacuolar changes and Civatte bodies. The dermal inflammatory infiltrate consisted of eosinophils, a few neutrophils along with dermal oedema [Table/Fig-4].
Bullous lesion of right hand
Bullous lesion on instep of right foot
Bullous lesion on instep of left hand
Acute interface dermatitis with prominent vacuolar change and individual necrotic keratinocytes within the epidermis
DISCUSSION
Way back in 1889, Bourns described a series of sharply demarcated hyperpigmented lesions on the lips and tongue of a patient after oral ingestion of 20 g of antipyrine. Years later, Brocq coined the French term, eruption erythemato-pigmentee fixe, meaning “fixed drug eruption” [12].The most characteristic findings of a fixed drug eruption are the recurrence of similar lesions at the same sites that heal with residual hyperpigmentation which may persist for months and years. The residual hyperpigmentation serves as an indicator of site recognition. The diagnosis of fixed drug eruption is not always easy; as in the case of nonpigmenting fixed drug eruptions, which do not have any residual hyperpigmentation. The development of molecular biology may help to unfold the exact pathogenesis of fixed drug eruptions, but as of now the reason for their recurrence on the same sites is still unknown. Importantly, the causative drug and cross-reactants should be avoided to prevent recurrence. Till date, rechallenge is the most reliable method of identifying causative drugs, but increasingly the use of skin tests has gained the attention of many investigators [13]. A genetic susceptibility to developing a fixed drug eruption with an increased incidence of HLA-B22 has been reported [14, 15].
Ciprofloxacin, the drug in question in the present case is in use since 1986 [15]. The drug has good tolerability profile and finds extensive usage in more than 56 countries. Due to its broad spectrum activity against gram positive and gram negative organisms, excellent tissue penetration, good results in skin and soft tissue infections and easy dosage schedule; ciprofloxacin remains the most popular antibacterial among the dermatologists. Nonetheless, the drug is not exempted form adverse reactions like rashes and photosensitivity. A single case of fixed drug eruption (FDE) due to ciprofloxacin has been reported earlier in a Japanese patient [16]. A novel case of bullous FDE in a twenty year old Indian pharmacist way back in 1995 has been reported [17]. Another case report of a similar bullous fixed drug eruption due to the drug in a 57 years old female has been on the records [18].
[1]. Krahenbuhl-Melcher A, Schlienger R, Lampert M, Haschke M, Drewe J, Krahenbuhl S, Drug-related problems in hospitals: a review of the recent literatureDrug Saf 2007 30(5):379-407. [Google Scholar]
[2]. Mahboob A, Haroon TS, Drugs causing fixed eruptions: a study of 450 casesInt J Dermatol Nov 1998 37(11):833-8. [Google Scholar]
[3]. Ozkaya-Bayazit E, Specific site involvement in fixed drug eruptionJ Am Acad Dermatol Dec 2003 49(6):1003-7. [Google Scholar]
[4]. Ozkaya-Bayazit E, Bayazit H, Ozarmagan G, Drug related clinical pattern in fixed drug eruptionEur J Dermatol Jun 2000 10(4):288-91. [Google Scholar]
[5]. Fischer G, Vulvar fixed drug eruption. A report of 13 casesJ Reprod Med Feb 2007 52(2):81-6. [Google Scholar]
[6]. Gupta S, Gupta S, Mittal A, David S, Oral fixed drug eruption caused by gabapentinJ Eur Acad Dermatol Venereol Feb 19 2009 [Google Scholar]
[7]. Katoulis AC, Bozi E, Kanelleas A, Psoriasiform fixed drug eruption caused by nimesulideClin Exp Dermatol Oct 2009 34(7) [Google Scholar]
[8]. Ronnau Ac, Sachs B, Von Schmiedeberg S, Hunzelmann N, Ruzicka T, Gleichmann E, Cutaneous adverse reaction to ciprofloxacin: Demonstration of specific lymphocyte proliferation and cross reactivity to ofloxacin in vitroActa Dermatol Venereol 1997 77:285-8. [Google Scholar]
[9]. Wilton L V, Pearce G L, Mann R D, A comparison of ciprofloxacin, norfloxacin, ofloxacin, azithromycin and cefixime examined by observational cohort studiesBr J Clin Pharmacol 1996 41:277-84. [Google Scholar]
[10]. Fluoroquinolone-induced generalized fixed drug eruption Jonathan L Hager MD, Mohsin R Mir MD, Sylvia Hsu MDDermatology Online Journal December 2009 15(12):8 [Google Scholar]
[11]. Campi P, Pichler WJ, Quinolone hypersensitivityCurr Opin Allergy Clin Immunol 2003 3:275-81. [Google Scholar]
[12]. Nordlund J J, Boissy R E, Hearing V J, King R A, Portonne J, The Pigmentary System: Physiology and Pathophysiology 1998 New YorkOxford Univ. Press [Google Scholar]
[13]. Lee Ai-Young, American Journal of Clinical Dermatology September/October 2000 1(5):277-285. [Google Scholar]
[14]. Pellicano R, Ciavarella G, Lomuto M, Di Giorgio G, Genetic susceptibility to fixed drug eruption: evidence for a link with HLA-B22J Am Acad Dermatol Jan 1994 30(1):52-4. [Google Scholar]
[15]. Pellicano R, Lomuto M, Ciavarella G, Di Giorgio G, Gasparini P, Fixed drug eruptions with feprazone are linked to HLA-B22J Am Acad Dermatol May 1997 36(5 Pt 1):782-4. [Google Scholar]
[16]. Kawada A, Hiruma M, Morimoto K, Fixed drug eruption induced by ciprofloxacin followed by ofloxacinContact Dermatitis 1994 31:182-3. [Google Scholar]
[17]. Bose KS, Ciprofloxacin-induced bullous fixed drug eruptionIndian J Dermatol Venereol Leprol [serial online] 1995 61:238-9.[cited 2012 Mar 5] [Google Scholar]
[18]. Ada S, Yilmaz S, Ciprofloxacin-induced generalized bullous fixed drug eruptionIndian J Dermatol Venereol Leprol. [serial online] 2008 74:511-2.[cited 2012 Mar 5] [Google Scholar]