The Aetiology of the Bloodstream Infections in the Patients Who Presented to a Tertiary Care Teaching Hospital in Kathmandu, Nepal
Santwana Pandey1, Shahid Raza2, Chandra Prakash Bhatta3
1 Lecturer, Department of Microbiology, Kantipur Dental College & Teaching Hospital, Kathmandu, Nepal.
2 Lecturer, Department of Microbiology, Kathmandu Medical College Teaching Hospital, Kathmandu, Nepal.
3 Associate Professor, Department of Microbiology, Kathmandu Medical College Teaching Hospital, Kathmandu, Nepal.
NAME, ADRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Ms. Santwana Pandey, Lecturer, Department of Microbiology Kantipur Dental College and Teaching Hospital Basundhara -3, Nepal.
Phone: 0977- 9841959893
E-mail: Santwana11@gmail.com
Background: Bloodstream infections are associated with a significant patient morbidity and mortality. The detection of microorganisms in the patients’ blood has a great diagnostic and prognostic significance. The early positive results provide valuable diagnostic information, based on which the appropriate antimicrobial therapy can be initiated.
Objective: To know the aetiology of the bloodstream infections in the Kathmandu Medical College, Nepal and the antibiotic sensitivity patterns of the causative organisms.
Materials and Methods: The blood specimens which were received from May 2010 to October 2010 in Kathmandu Medical College and Teaching Hospital, Kathmandu, Nepal, were processed and all the positive isolates were included in the study. The isolates were identified by the standard laboratory procedures. The antibiotic susceptibility patterns were determined by the modified Kirby Bauer antibiotic sensitivity method.
Result: Of the 1089 blood cultures which were received with the suspected cases of blood stream infections, 138 (12.6 %) were bacteriologically positive. Salmonella serotypes were isolated in 42.7% cases of blood stream infections, followed by Klebsiella pneumoniae in 19.5%, Staphylococcus aureus in 15.9% and others in the rest of the cases. All the gram-negative bacilli isolates showed lower degrees of resistance to amikacin and ofloxacin. All the gram positive isolates were sensitive to amikacin, oxacillin and vancomycin.
Conclusion: This study stresses on the need for a continued screening and surveillance in the routine blood culture technique for starting with the empiric therapy for blood borne infections.
Bloodstream infection, Blood culture, Antibiotic susceptibility, Salmonella spp
INTRODUCTION
Blood culture is the most significant specimen type which is used for the diagnosis of Blood Stream Infections (BSIs). Bacteriological culture is done to isolate the offending pathogens and to know about the sensitivity pattern of the isolates. It remain the mainstay of definitive diagnosis and the management of BSIs [1]. Respiratory, genitourinary tract and intra-abdominal foci are often the identifiable sources of the bloodstream infections [2].
BSIs are one of the main causes of death in hospitalized patients, with mortality rates between 30-70% [3]. Blood cultures also provide essential information for the evaluation of a variety of diseases like endocarditis, pneumonia, and pyrexia of unknown origin and particularly, in patients with suspected sepsis. The microorganisms which are present in the circulating blood, whether continuously or intermittently, are a threat to the host [4].
A variety of organisms are isolated in BSIs. Organisms like Staphylococci, Enterococci and Enterobacteriaceae are often implicated. Presently, there is an increase in the incidence of the bacteremia which is caused by the members of Enterobacteriaceae and other gram negative bacilli like Psedumonas spp and Acinetobacter spp [5].
Gram negative bacteremia can result in septic shock and the mortality is even greater with high-grade bacteremia and polymicrobial infections. Gram positive bacteremia is also seen to Bhattabe on the rise, especially among neonates and children [6]. The bacteremia which is caused by the Enterobacteriaceae family is associated with an increased mortality as compared to the BSIs caused by Gram-positive bacteria [7]. Furthermore the presence of multidrug resistance to these strains leads to a longer hospital stay, more expensive/ toxic drugs and a higher mortality.
Hence, an early initiation of the appropriate antimicrobial treat-ment is critical in decreasing the morbidity and the mortality among the patients with BSIs. The potential for antimicrobial resistance is also one of the considerations of the physicians when they select a regimen to treat the patients, especially those with bacteremia and septicaemia. Hence, the formulation of a common bacteriological profile and antimicrobial susceptibility patterns will guide the choice of the empiric antimicrobial regimens for these patients. This study amis to report the pattern of the bacterial isolates in the blood and their antibiograms, as were seen in a tertiary care hospital in Kathmandu, Nepal.
MATATERIALS AND METHODS
The present study was carried out between May 2010 and October 2010 in the Department of Microbiology, Kathmandu Medical College, Kathmandu, Nepal. The samples was collected from hospitalized patients and from the patients who attended different OPDs with the symptoms of BSIs. One ml (neonates) and 5 ml (children and adults) of blood was collected and it was inoculated into 10 and 50 ml, respectively, of brain heart infusion broth (1:10 dilution). The culture bottles were incubated at 37°C aerobically. Periodic subcultures were done onto Mac Conkey’s agar and blood agar after an overnight incubation and after 48 hours and 72 hours. The growth which was obtained was identified by the colony characteristics, gram staining and biochemical tests [8].
The antibiotic susceptibility test which helps in studying the susceptibilities of the isolates to routine antibiotics, was done by modified Kirby-Bauer method. The zone sizes were measured and interpreted according to the CLSI standards [9].
RESULT
Of the 1089 blood cultures which were sent with suspected cases of BSIs, 138 (12.6%) were bacteriologically positive. Among the pathogens which were isolated, 116 (84%)were gram-negative bacilli and 22 (16%) were gram-positive cocci [Table/Fig-1]. Among the gram-negative bacilli, all the isolates belonged to the family, Enterobacteriaceae.
Distribution of Bacterial Pathogens In blood culture
| Organism | Number | Percentage |
|---|
| Salmonella serotypes | 59 | 42.75 |
| Klebsiella pneumoniae | 27 | 19.56 |
| Staphylococcu aureus | 22 | 15.94 |
| Enterobacter spp | 16 | 11.59 |
| Proteus spp | 6 | 4.34 |
| E. coli | 5 | 3.62 |
| Citrobacter spp | 3 | 2.17 |
| Total | 138 | 100 |
The Salmonella serotypes were isolated in 42.7% cases of BSIs, followed by Klebsiella pneumonia (19.5%), S. aureus (15.9%) and others [Table/Fig-1]. All the gram negative isolates showed higher susceptibilities to amikacin and ofloxacin. All the isolates of Staphylococcus aureus were sensitive to oxacillin and vancomycin. [Table/Fig-2 and 3]
Antibiotic Resistance Patterns of Gram Negative Bacteria
| Antibiotic tested | Salmonella serotype n=59(%) | Klebsiella pneumoniae n=27 (%) | Enterobacter spp n=16(%) | Proteus spp n=6(%) | E,coli n=5(%) | Citrobacter spp n=3(%) | Total n= 138(%) |
|---|
| Amikacin | 2 (3.3) | 0 (0.00) | 0 (0.0) | 3 (50) | 1 (20) | 2 (66.6) | 8 (5.7) |
| Ciprofloxacin | 5 (8.4) | 2 (7.4) | 0 (0.0) | 1 (16.6) | 1 (20) | 2 (66.6) | 11 (7.9) |
| Ofloxacin | 2 (3.3) | 1 (3.7) | 2 (12.5) | 0 (0.0) | 1 (20) | 2 (66.6) | 8 (5.7) |
| Ceftriaxone | 6 (10.1) | 1 (3.7) | 3 (18.7) | 4 (66.6) | 2 (40) | 2 (66.6) | 18 (13) |
| Cefuroxime | 14 (23.72) | 2 (7.4) | 4 (25) | 3 (50) | 3 (60) | 2 (66.6) | 28 (20.2) |
| Chloramphenicol | 3 (5.0) | 2 (7.4) | 2 (12.5) | 0 (0.0) | 3 (60) | 2 (66.6) | 12 (8.6) |
| Ampicillin | 17 (28.8) | 6 (22.2) | 7 (43.7) | 5 (83.3) | 4 (80) | 3 (50) | 42 (30.4) |
| Gentamicin | 8 (2.63) | 1 (3.7) | 4 (25) | 4 (66.6) | 2 (40) | 2 (66.6) | 21 (15.2) |
Antibiotic Resistance Pattern of Gram Positive Bacteria*
| Antibiotic | Resistance n=22(%) |
|---|
| Penicillin | 22(100) |
| Oxacillin | 0(00) |
| Vancomycin | 0(00) |
| Gentamicin | 4(18.1) |
| Amikacin | 5(22.7) |
| Co-trimoxazole | 10(45.5) |
| Ciprofloxacin | 3(13.6) |
DISCUSSION
Many studies have demonstrated that a rapid antimicrobial therapy which is administered against blood stream infections can reduce the morbidity and the mortality rate [10]. Though the clinical feature may be useful to identify the causes of the blood stream infections, the culture method is the gold standard technique for identifying the causative organisms and for establishing their antibiotic profiles.
We are reporting a 12.6% blood culture positivity rate. A study which was conducted in western Nepal in 2007 showed the isolation rate to be 10.28% [11], while a similar study which was done in Kavre had lower rate of 6.9% [12]. A similar study which was done in Iran also showed a lower positivity rate of 5.6% [13]. A very recent study done in south India also showed 8.39% culture positive samples [14]. Similarly, an Ethiopian study also showed an 8.8% blood culture positivity rate [15]. In contrast to the rates which have been mentioned above, studies which were conducted in Mangalore, Delhi [6, 16] and Pakistan [17, 18] showed markedly higher rates (of more than 20%).
In comparison, the isolation rates were found to be especially low in Nepal. This was because most of the patients in Nepal were on antibiotics before they had come to the tertiary care hospital. The variation in the blood culture positivity was also related to different factors such as the numbers of blood culture, volume of the blood taken and the types of culture broth used [16].
In the current study, a higher incidence of gram negative organisms was found against gram positive cocci. Similar results were also seen in a study which was conducted in the western part of Nepal [11] and Kavre [12]. The studies which were conducted in the Indian subcontinent like India [6,14,16] and Pakistan [19] also showed concurrent results. But the studies which were done in Europe and USA showed higher yields of the gram positive growth [20].
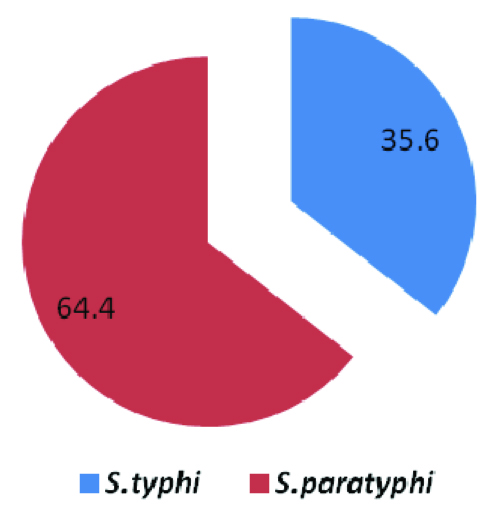
Salmonella spp, K. pneumoniae and S. aureus were the most common aetiological agents of the BSIs in our study [Table/Fig-1]. A high prevalence of the Salmonella spp was seen in the various studies which were done in Nepal [11, 12]. A study which was done in the western hilly region of Nepal reported that enteric fever accounted for 56.8% of all the patients who presented with fever in a hospital [21]. This indicated that the Salmonella spp were important aetiological agents of the blood stream infections in Nepal. The isolation rate of S. Paratyphi A (64.4%) followed that of S. Typhi (35.6%) in our study, which was in agreement to previous reports from western Nepal [11] [Table/Fig-4]. This does not have any implications for the therapy, but it has implications for vaccination strategies, as the current typhoid vaccines do not protect against paratyphoid fever.
Percentage of enteric salmonella isolates
In this study, the Salmonella spp which are the important cause of the community-acquired BSIs, were noted to be susceptible to most of the routinely used antibiotics. But more than 20% showed resistance to the first line drugs such as cefuroxime and ampicillin. However, all the Salmonella isolates were sensitive to chloramphenicol; which was attributed to the long-term disuse of the above mentioned drug.
Coagulase-positive Staphylococci were isolated during this study, from 22 patients. The resistances of S. aureus to erythromycin, gentamicin, amikacin and ciprofloxacin were 45.5%, 18.1%, 22.7% and 15%, respectively. None were Methicillin resistant Staphylococcus aureus and all the strains were sensitive to vancomycin. A study which was done by Mehdinejad M et al., also reported cent percent sensitivity for Vancomycin [13].
We observed that all the members of the family of gram negative bacilli were frequently resistant to the first-line antibiotics such as ampicillin, cefuroxime and gentamicin [Table/Fig-2]. All the gram negative isolates from a similar study which was conducted in South India also showed an increased resistance for ampicilin [14]. About 18% of the isolates were also resistant to the extended-spectrum cephalosporins (ceftriaxone). This type of resistance is a marker for the presence of Extended Spectrum Beta Lactamases (ESBLs) [22].This is a very alarming development as the mortality is much higher with the ESBL producing Enterobacteriaceae [23].
The changing spectrum of microbial pathogens within the hospital environment and the alarming microbial resistance to the antibiotic drugs confirm the need of local surveillance programs and national programs to monitor the prevalence of bacterial resistance. The data which is received from the surveillance programs can be used as an important information for understanding the pattern of antibiotic resistance. Which can also encourage the physicians to discontinue the misuse of antibiotics, which remains the key point in controlling the occurrence and the spread of resistance.
CONCLUSION
As the degree of the antibiotic resistance rate for the blood stream pathogens is alarming, it is mandatory to monitor the susceptibility of these isolates, in order to avoid the inappropriate use of antibiotics in hospitals. It is obviously clear that this purpose can really be achieved only by maintaining a close correlation between the physicians and the laboratory.
[1]. Jain A, Roy I, Gupta MK, Kumar M, Agrawal SK, Prevalence of extended-spectrum β-lactamase-producing Gram-negative bacteria in septicaemic neonates in a tertiary care hospitalJ Med Microbiol 2003 52:421-25. [Google Scholar]
[2]. Jarvis WR, The evolving world of healthcare-associated bloodstream infection surveillance and prevention: is your system as good as you think?Infect Control Hosp Epidemiol 2002 23:236-38. [Google Scholar]
[3]. Vincent JL, Bihari DJ, Suter PM, Bruining HA, White J, Nicolas-Chanoin MH, The prevalence of nosocomial infection in intensive care units in Europe: Results of the European Prevalence of Infection in Intensive Care (EPIC) StudyJAMA 1995 274:639-44. [Google Scholar]
[4]. Yagupsky P, Nolte FS, Quantitative aspects of septicaemiaClin Microbiol Rev 1990 3:269-79. [Google Scholar]
[5]. Karpuch J, Azzizi I, Beer S, Bacteremia among hospitalized children- a four years prospective studyJ Infect 1984 9:139-42. [Google Scholar]
[6]. Prabhu K, Bhat S, Rao S, Bacteriologic profile and antibiogram of blood culture isolates in a pediatric care unitJ Lab Physicians 2010 2:85-88. [Google Scholar]
[7]. Diekema DJ, Pfaller MA, Jones RN, Doern GV, Winokur PL, Gales AC, Survey of bloodstream infections due to gram-negative bacilli: frequency of occurrence and antimicrobial susceptibility of isolates collected in the United States, Canada, and Latin America for the SENTRY antimicrobial surveillance program, 1997Clin Infect Dis 1999 29:595-607. [Google Scholar]
[8]. Watt B, Miles RS, Collee JG, Tests for identification of bacteriaPractical Medical Microbiology 1996 14thNew YorkChurchil Livingstone:131-50. [Google Scholar]
[9]. Clinical Laboratory Standards Institute (CLSI)Performance standards for antimicrobial disk susceptibility tests. Approved Standard 2006 9thWayne PA, USACLSI document M2-A9. CLSI [Google Scholar]
[10]. Harbarth S, Garbino J, Pugin J, Romand JA, Lew D, Pittet D, Inappropriate initial antimicrobial therapy and its effect on survival in a clinical trial of immunomodulating therapy for severe sepsisAm J Med 2003 115:529-35. [Google Scholar]
[11]. Easow MJ, Joseph MN, Dhungel AB, Chapagain B, Shivananda PG, Blood Stream Infections among febrile patients attending a teaching hospital in Westren Region of NepalAMJ 2010 3(10):633-37. [Google Scholar]
[12]. Sharma NP, Peacock SJ, Phumratanaprapin W, Day N, White N, Pukrittayakamee S, A hospital-based study of bloodstream infections in febrile patients in Dhulikhel Hospital Kathmandu University Teaching Hospital, NepalSoutheast Asian J Trop Med Public Health 2006 37:351-56. [Google Scholar]
[13]. Mehdinejad A, Khosravi D, Morvaridi A, Study of prevalence and antimicrobial susceptibility pattern of bacteria isolated from blood culturesJ Bio Sci 2009 9:249-53. [Google Scholar]
[14]. Vanitha RN, Kannan G, Venkata NM, Vishwakanth D, Nagesh VD, Yogitha M, A retrospective study on blood stream infections and antibiotic susceptibility patterns in a tertiary care teaching hospitalInt J Pharm Pharm Sci 2012 4:543-48. [Google Scholar]
[15]. Zenebe T, Kannan S, Yilma D, Beyene G, Invasive bacterial pathogens and antibiotic susceptibility patterns in University associated specialized hospital in southeast EthopiaEthiop J Health Sci 2011 21:1-8. [Google Scholar]
[16]. Mehta M, Dutta P, Gupta V, Antimicrobial susceptibility pattern of blood isolates from a teaching hospital in north IndiaJpn J Infect Dis 2005 58:174-76. [Google Scholar]
[17]. Chaudhry I, Chaudhry NA, Munir M, Hussain R, Tayyab M, Etiological Pattern of septicemia at Three Hospitals in LahoreJCPSP 2000 10:375-79. [Google Scholar]
[18]. Latif S, Anwar MS, Ahmad I, Bacterial pathogens responsible for blood stream infection(BSI) and pattern of drug resistance in tertiary care hospital in LahoreBiomedia 2009 25:101-05. [Google Scholar]
[19]. Mahmood A, Blood stream infections in a medical intensive care unit: spectrum and antibiotic susceptibility patternJ Pak Med Assoc 2001 51:213-15. [Google Scholar]
[20]. Siefert H, Wisplinghoff H, Bloodstream infection and endocarditisTopley and Wilson’s Microbiology and Microbial Infections 2005 110thLondonEdward Arnold:509-26. [Google Scholar]
[21]. Biswas R, Dhakal B, Das RN, Shetty KJ, Resolving diagnostic uncertainty in initially poorly localizable fevers: a prospective studyInt J Clin Pract 2004 58:26-28. [Google Scholar]
[22]. Nathisuwan S, Burgess DS, Lewis II JS, Extended-Spectrum β-Lactamases: epidemiology, detection, and treatmentPharmacotherapy 2001 21:920-28. [Google Scholar]
[23]. Blomberg B, Jureen R, Manji KP, Tamim BS, Mwakagile DS, Urassa WK, High Rate of Fatal Cases of Pediatric Septicemia Caused by Gram-Negative Bacteria with Extended-Spectrum Beta-Lactamases in Dar es SalaamTanzania JCM 2005 43:745-49. [Google Scholar]