Background: Adenosine Deaminase (ADA) has been suggested to be an important enzyme which is associated with the cell mediated immunity, but its clinical significance in typhoid fever has not yet been characterized. The present study was taken up to evaluate the serum ADA activity in patients of typhoid fever. The levels of ADA were also measured in the patients who were suffering from other febrile illnesses.
Material and Method: This was a case control study. The subjects who were included in this study were divided into 3 groups. Group A consisted of 50 normal healthy individuals who served as the controls. Group B consisted of 50 patients, both males and females of all age groups, who were suffering from culture positive typhoid fever. Group C consisted of 50 patients who were suffering from febrile illnesses other than typhoid fever like viral fever, gastro enteritis, malaria, tonsillitis, upper respiratory tract infections, etc. The serum levels of ADA were estimated in all the subjects who were under study.
Results: The serum ADA level was found to be increased in the patients of typhoid fever as compared to that in those with other febrile illnesses and in the controls.
Conclusion: From the present study, it can be concluded that there was a statistically significant increase in the serum ADA levels in the patients with typhoid.
INTRODUCTION
Typhoid fever is an important public health problem in India. It is a life-threatening systemic infection which is caused by the bacterium, Salmonella enterica serotype typhi. It is a highly adapted, human-specific pathogen which occurs more frequently in the underdeveloped regions of the world, where overcrowding and poor sanitation are prevalent [1].
Typhoid is usually acquired through the ingestion of water or food which is contaminated by the urine or the faeces of infected carriers and, as such, it is a common illness in the areas where the sanitation is poor [1]. One of the most famous carriers was Typhoid Mary, a cook who infected at least 51 people [2]. Outbreaks of typhoid fever occur most often in the developing countries, in refugee camps and in overwhelmed areas with a high population density. In some areas, the annual incidence is as high as 198 cases per 1,00,000 [3] and, this disease causes considerable morbidity in children [4]. Worldwide, at least 17 million new cases and up to 6,00,000 deaths are reported annually [1].
The severity and the clinical outcome of typhoid are controlled by the duration of the illness before the initiation of the appropriate therapy [5, 6]. The case-fatality rate of typhoid fever is 10%, but it can be reduced to 1% with the appropriate antibiotic treatment [1]. Hence, an early diagnosis and the initiation of the therapy are important in decreasing the morbidity and the mortality which are caused due to the typhoid infection.
Adenosine deaminase, an enzyme, which is present in the red cells and the vessel wall, catalyses the irreversible hydrolytic deamination of adenosine to inosine and 2’-deoxyadenosine to 2’-deoxyinosine. Inosine and 2’-deoxyinosine are converted to hypoxanthine, xanthine and finally, to uric acid [7]. ADA is considered as a good marker of the cell mediated immunity [8]. The structure of ADA is shown in [Table/Fig-1]. The high lymphocyte ADA activities were found to be elevated in the diseases in which there was a cell mediated immune response [9].
O.P. Mishra and his colleagues [10] reported that the Adenosine deaminase (ADA) enzyme was required for lymphocyte proliferation and differentiation [11].
J.EJ. Ungerer et al., concluded that an increase in the ADA values in the serum of typhoid patients implied that the increase possibly originated from the monocytes.
Three-dimensional representation of ADA structure
Kumar et al., stated that only a little information was available on the development of the specific Cell-Mediated Immune Response (CMIR) and on its role in the protection from this disease. It was shown that the specific CMIR developed in a majority of the patients with typhoid fever [12, 13].
Even though there are reports which are available on the serum ADA levels in patients of typhoid fever, these are not very clear and conclusive.
Hence, in the light of above mentioned facts, the present study was designed to evaluate the serum ADA activity in patients of typhoid and it was compared with the ADA activity in patients with other febrile illnesses and in the controls.
MATERIALS AND METHODS
The subjects who were included in the present study were 100 outpatients who were suffering from fever of <102°F of less than 7 days duration, from the Department of Medicine at Osmania General Hospital, Hyderabad, and from the Sir Ronald Ross Institute of Tropical Medicine, Hyderabad, India, who attended these institutions over a period of 2 years, from April 2008 to April 2010. A group of 50 normal healthy individuals who were age and gender matched, served as the controls and were grouped as Group A. Blood cultures were put up of the samples from fever patients. The culture positive patients were grouped under Group B and the culture negative patients were lined up under Group C. This study was done in compliance with the ethical guidelines of the institute.
These 150 subjects were divided into 3 groups:
GROUP A comprised of 50 normal healthy individuals, both males and females of all ages, from the general population who volunteered for getting included in the present study.
GROUP B comprised of 50 patients who were suffering from typhoid, both males and females of all ages, whose blood samples showed positivity for the culture of the typhoid bacilli.
GROUP C comprised of 50 patients who were suffering from fevers which were other than typhoid, both males and females of all ages, whose blood samples showed negativity for the culture of the typhoid bacilli.
Informed consents were taken from all the subjects who were included in the study. This was an open-label, non-interventional study and a case control study. The serum ADA levels were estimated by the Giusti and Galanti method [14, 15], in all the three groups. As a precaution, to minimize the technical error in the study, all the reagents which were used for the estimation were prepared with double distilled water, to prevent the interference of the ammonia content in the tap water.
The cases with a history of AIDS, Diabetes Mellitus, collagen vascular disease, and liver disorders, were not included in the study.
STATISTICS
The results were represented as tables and bar diagrams. The statistical analysis was carried out by using the SPSS (Statistical Package for social science) software, version 15.0 [16].
The data was expressed by using the mean and the standard deviation of ADA in the different groups. The multiple comparison ANOVA test was used to assess the significance of the difference of the means between Group A, Group B and Group C. The independent sample ‘t’ test and the ‘p’ value were used to assess the significance of the difference of the means between the cases and the controls. A p value of < 0.05 was considered as significant.
The specificity and the sensitivity of the serum ADA levels in diagnosing typhoid fever were computed by using ROC curves with the “graph pad prism” software.
RESULTS
The statistical analysis showed that the age and sex distribution in these three groups was comparable [Table/Fig-2] and [Table/Fig-3].
Case distribution in relation to age
| Group | Controls | Cases |
|---|
| Group A | Group B | Group C |
|---|
| Age <15 yrs | 30 | 28 | 33 |
| Age <15 yrs | 20 | 22 | 17 |
| Total | 50 | 50 | 50 |
Case distribution in relation to Gender
| Group | Group A | Cases |
|---|
| Group B | Group C |
|---|
| F | 25 | 25 | 25 |
| M | 25 | 25 | 25 |
| Total | 50 | 50 | 50 |
Care was taken to eliminate bias by distributing 150 subjects evenly into 3 groups with respect to age and gender.
The mean values for serum ADA are significantly higher in the typhoid fever group as compared to those of the other fever group and the control group, as shown in [Table/Fig-4,5 & 6].
Mean and SD values of Serum ADA (U/L) in 3 groups.
| Parameter | Group A (n=50) | Group B (n=50) | Group C (n=50) |
|---|
| Mean+/-SD | Mean+/-SD | Mean+/-SD |
|---|
| Serum ADA (U/L) | 15.00+/-5.051 | 38.00+/-9.326 | 21.54+/-5.128 |
ANOVA Multiple Comparison of significance between the 3 groups.
| Parameter | Significance in between Groups | Significance |
|---|
| ADA | 0.000 | Yes |
The ‘p’ values between the groups were < 0.05 for ADA. So, posthoc tests were done for this parameter [Table/Fig-6].
| Dependent Variable | (I) | (J) | Mean Difference (I-J) | Sig. |
|---|
| ADA (U/L) | Group A | Group C | -6.540(*) | 0.000 |
| Group B | -23.000(*) | 0.000 |
| Group B | Group A | 23.000(*) | 0.000 |
| Group C | 16.460(*) | 0.000 |
| Group C | Group A | 6.540(*) | 0.000 |
| Group B | -16.460(*) | 0.000 |
*The mean difference is significant at the .05 level.
In order to assess the maximum sensitivity and the specificity of the various parameters in identifying the abnormalities, the best cut off values were calculated by using the ROC analysis. The best cut off values were established by selecting a point which was closer to top left hand curve that provided the greatest sum of the sensitivity and the specificity.
From the above [Tables/Fig-7, 8, 9, 10 & 11], it was seen that the rise in the serum ADA values in the typhoid group was statistically significant as compared to those of the control group and the other fever group.
Sensitivity specificity at best cut off values in between 3 groups
| Best cutoff value | Sensitivity | Specificity |
|---|
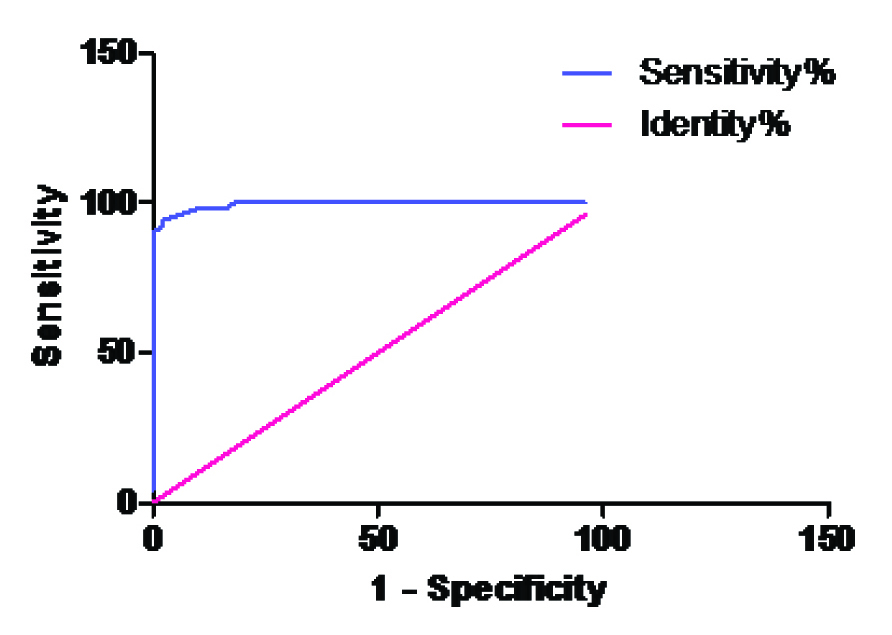
| Group A Vs. Group B | 23.5 | 94% | 98% |
| Group B Vs. Group C | 27.5 | 86% | 92% |
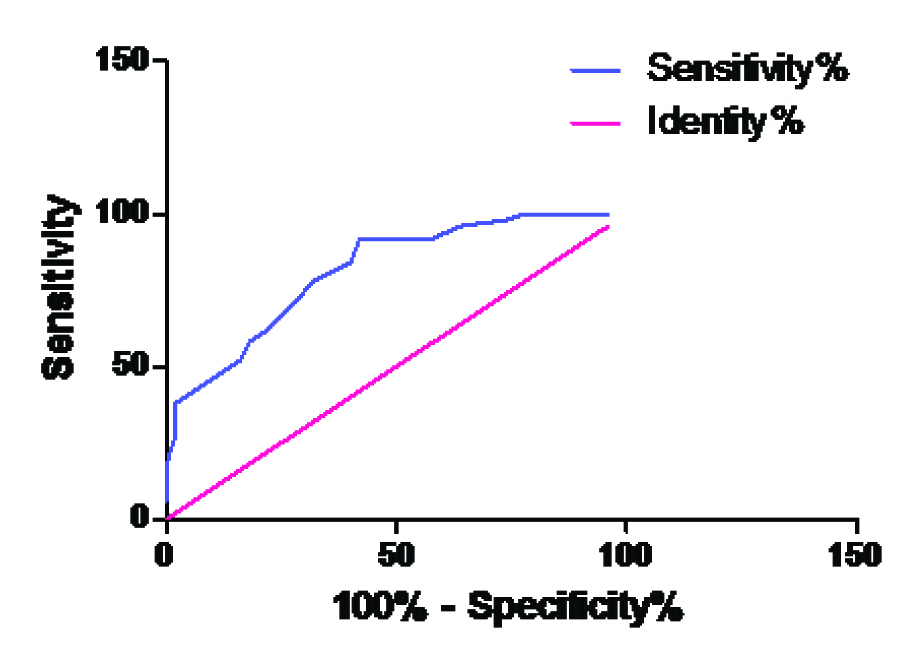
| Group A Vs. Group C | 15.5 | 92% | 58% |
Table comparing AUC, 95% CI and p value among the 3 groups
| Parameters | AUC | 95% CI | p value |
|---|
| A&B AUC | 0.9936 | 0.9844 to 1.003 | < 0.0001 |
| B&C AUC | 0.9432 | 0.9018 to 0.9846 | < 0.0001 |
| A&C AUC | 0.815 | 0.7339 to 0.8961 | < 0.0001 |
ROC curve for ADA in Group A Vs Group C
ROC curve for ADA in Group A Vs Group B
ROC curve for ADA in Group C Vs Group B
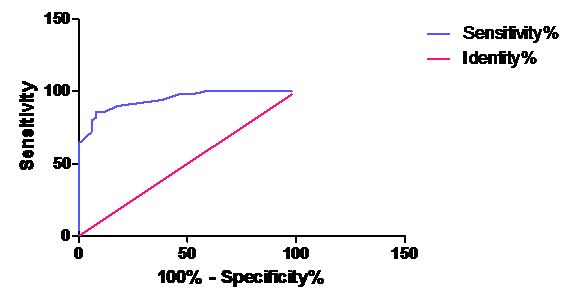
The best cut off values for ADA, along with the sensitivity and the specificity for the cases, have been presented in [Table/Fig-7]. The area under the curve of ADA is shown in [Table/Fig-8]. These tables provide the unbiased estimates of the sensitivity and the specificity; it is a comprehensive representation of the pure accuracy i.e. the discriminating ability over the entire range of the test. It doesn’t require the selection of a particular decision threshold because the whole spectrum of the possible decision threshold is included.
In the present study, the best cut off values of ADA were calculated by using the sensitivity and the specificity from the ROC curves and its discriminatory capacity was represented as the values of the area under the curve.
Thus, serum ADA showed the highest sensitivity and specificity in discriminating the cases from the controls. Hence, serum ADA is the best discriminatory marker.
DISCUSSION
Typhoid is a severe, contagious and a life-threatening disease. It is transmitted through food, drinks and water which are contaminated with the bacteria, Salmonella typhi, which may result in fever with severe complications.
Typhoid fever is endemic in India. A limited study which was done in an urban slum in India showed that 1 per cent of the children of up to 17 years of age suffered from typhoid fever every year [17, 18].
The patho-physiology of typhoid fever is a complex process, which proceeds through several stages [19].
The often nonspecific symptoms of typhoid fever result in frequent diagnostic confusions with malaria, dengue fever, influenza or other febrile illnesses. A confirmed diagnosis requires the isolation of S. typhi in the laboratory through blood cultures or occasionally, through bone marrow cultures. The latter has been shown to be approximately 50% more sensitive than the blood cultures. However, these tests are not conducted for a majority of the patients in the developing countries, especially those who are treated in non-hospital settings [20].
The current serological diagnostic assays for typhoid fever are based on detecting the antibodies against the Salmonella species, which result in a high false-positive rate [21]. The newer methods of diagnosis, such as the use of DNA probes and the polymerase chain reaction, to detect S.typhi directly in the blood, are now available, but their use in endemic areas is limited [22].
In the present study, the evaluation of adenosine deaminase was done for the purpose of selecting the markers with a high sensitivity and specificity. All the Salmonella infections begin with the ingestion of the organisms from contaminated food or water. Once the Salmonellae reach the small intestine, they penetrate the mucous layer of the gut and traverse the intestinal layer through the phagocytic micro fold (M) cells that reside within the Peyer’s patches. After crossing the epithelial layer of the small intestine, they are phagocytosed by the macrophages. Once they are phagocytosed, the Salmonellae disseminate throughout the body in the macrophages via the lymphatics and colonize the reticuloendothelial tissues (liver, spleen, lymph nodes, and bone marrow). This results in the secretion of cytokines by the macrophages and the epithelial cells, in response to the bacterial products that are recognized by the innate immune receptors, when a critical number of organisms have replicated. Over time, the development of hepatosplenomegaly is likely to be related to the recruitment of the mononuclear cells and the development of a specific acquired cell-mediated immune response to the S. typhi colonization. The recruitment of additional mononuclear cells and lymphocytes to the Peyer’s patches during the several weeks after the initial colonization/infection can result in a marked enlargement and necrosis of the Peyer’s patches, which may be mediated by the bacterial products that promote cell death, as well as the inflammatory response [23].
This release of various substances leads to alterations in the concentrations of the many biological parameters in the body fluids. In the present study, we observed a statistically significant increase in the concentrations of the serum ADA levels in the typhoid fever patients as compared those in the other fever patients and in the healthy controls. This has also been reported by many others [24, 10, 25–31].
ADA is considered as marker of the cell mediated immunity. The raised level of the ADA activity under antigenic stimulation shows the importance of this enzyme in the rapid proliferation of cells, in order to prevent the accumulation of toxic metabolites. O.P. Mishra and his colleagues [10] reported that the Adenosine Deaminase (ADA) enzyme is required for lymphocyte proliferation and differentiation [11]. Its main biological activity is detected in the T-lymphocytes. Typhoid fever is associated with the development of the cell mediated immunity and thus, has increased values of serum ADA.
In conclusion, our study suggested that there was an increase in the serum ADA levels in the patients who were suffering from typhoid fever.
*The mean difference is significant at the .05 level.
[1]. Typhoid fever [fact sheet N149]. Geneva: World Health Organization; 1997. Available: www.who.int/inf-fs/en/fact149.html (accessed 2003 June 23) [Google Scholar]
[2]. Brooks J, The sad and tragic life of Typhoid MaryCMAJ 1996 154(6):915-16. [Google Scholar]
[3]. Lin FY, Vo AH, Phan VB, Nguyen TT, Bryla D, Tran CT, The epidemiology of typhoid fever in the Dong Thap Province, Mekong Delta region of VietnamAm J Trop Med Hyg 2000 62:644-48. [Google Scholar]
[4]. Sinha A, Sazawal S, Kumar R, Sood S, Reddaiah VP, Singh B, Typhoid fever in children aged less than 5 yearsLancet 1999 354:734-37. [Google Scholar]
[5]. Tharwat F, Ismail. Rapid diagnosis of typhoid feverIndian J Med Res April 2006 123:489-92. [Google Scholar]
[6]. Cohen JI, Bartlett JA, Corey GR, Extra-intestinal manifestations of Salmonella infectionsMedicine 1987 66:349-88. [Google Scholar]
[7]. Hoshino T, Yamada K, Masuoka K, Tsuboi I, Itoh K, Nonaka K, Elevated adenosine deaminase activity in the serum of patients with diabetes mellitusDiabetes Res Clin Pract 1994 Sep 25(2):97-102. [Google Scholar]
[8]. Sullivan JL, Oxborne WRA, Wedgewood RJ, Adenosine deaminase activity in lymphocytesBr J Haematol 1977 37:157-58. [Google Scholar]
[9]. Shiva Prakash M, Chennaiah S, Murthy YSR, Anjaiah E, Rao Ananda S, Suresh C, Altered adenosine deaminase activity in Type 2 diabetes mellitusJIACM 2006 7(2):114-17. [Google Scholar]
[10]. Mishra OP, Gupta BL, Ali Z, Nath G, Chandra L, Adenosine deaminase activity in typhoid feverIndian Pediatr 1994 Nov 31(11):1379-84. [Google Scholar]
[11]. Sullivan JL, Osborne WRA, Wedgwood RJ, Adenosine deaminase activity in lymphocytesBr J Hematol 1977 37:157-58. [Google Scholar]
[12]. Kumar R, Malaviya A N, Murthy R G S, Venkataraman M, Mohapatra L N, Immunoglobulins, C3, antibodies and leucocyte migration inhibition in patients with typhoid fever and TAB vaccinated individualsInfect. Immun 1974 10:1219 [Google Scholar]
[13]. Baker NM, Mills AE, Rachman I, Thomas JEP, Haemolytic-Uraemic Syndrome in Typhoid FeverBritish Medical journal 1974 2:84-87. [Google Scholar]
[14]. Giusti G, Adenosine deaminaseMethods of enzymatic analysis. In: Bergmeyer HU editor 1974 22ndNew YorkAcademic press inc.:1092-99. [Google Scholar]
[15]. Kaur Amandeep, Kukreja Sahiba, Malhotra Naresh, Neha Serum Adenosine Deaminase Activity and Its Correlation with Glycated Haemoglobin Levels in Patients of Type 2 Diabetes MellitusJournal of Clinical and Diagnostic Research 2012 April 6(2):252-56. [Google Scholar]
[16]. Supriya Mohanty Shrabani, Murgod Roopa, Pinnelli Venkata Bharat Kumar, Raghavendra DS, Hypomagnesemia, Lipid profile and Glycosylated haemoglobin in type 2 Diabetes Mellitus patientsIntl. J Chem Pharm Res 2012 1(5):116-23. [Google Scholar]
[17]. Sameera K, Sunitha T, Kumar Naveen P, Sujatha P, Evaluation of serum lactate dehydrogenase levels in typhoid feverInt J Pharm Bio Sci. 2013 Jan 4(1:(B)):944-50. [Google Scholar]
[18]. Agarwal PK, Gogia Atul, Gupta RK, Typhoid FeverJournal of Indian Academy of Clinical Medicine 2004 5(1):60-64. [Google Scholar]
[19]. Abro AH, Abdou AMS, Gangwani JL, Ustadi AM, Younis NJ, Hussaini HS, Ischemia modified albumin a potent marker in acute myocardial infarction in normolipidaemicPak J Med. Sci 2009 25(2):166-71. [Google Scholar]
[20]. WHO: Weekly epidemiological record (http://www.who.int/wer). 8 FEBRUARY 2008; 83rd YEAR, 83( 6): 49–60 [Google Scholar]
[21]. Liang L, Immune profiling with a Salmonella Typhi antigen microarray identifies new diagnostic biomarkers of human typhoidSci Rep 2013 3:1043doi: 10.1038/srep01043. Epub 2013 Jan 9 [Google Scholar]
[22]. Effa EE, Bukirwa H, Azithromycin for treating uncomplicated typhoid and paratyphoid fever (enteric fever)Indian Pediatr 2009 Jan 46(1):51-52. [Google Scholar]
[23]. Harrison’s Principles of Internal Medicine 16th Edition, The McGraw-Hill Companies, ISBN 0-07-140235-7 [Google Scholar]
[24]. Ungerer J.P.J., Burger H.M., Bissbort S.H., Vermaak W.J.H., Adenosine Deaminase Isoenzymes in Typhoid FeverEur. J. Clin. Microbiol. Infect. Dis:510-12. [Google Scholar]
[25]. Khosla S. N., Kumar D., Singh V., Lymphocytic adenosine deaminase activity in typhoid feversPostgrad Med J 1992 68:268-71. [Google Scholar]
[26]. Casanueva V, Cid X, Cavicchioli G, Oelker M, Cofre J, Chiang MT, Serum adenosine deaminase in the early diagnosis of typhoid feverPediatr Infect Dis J 1992 Oct 11(10):828-30. [Google Scholar]
[27]. Galanti B, Nardiello S, Russo M, Fiorentino F, Increased lymphocyte adenosine deaminase in typhoid feverScand J Infect Dis 1981 13(1):47-50. [Google Scholar]
[28]. Khosla SN, Kumar D, Singh V, Lymphocytic adenosine deaminase activity in typhoid feverPostgraduate Medical Journal 1992 68:268-71. [Google Scholar]
[29]. Russo M, Pizzella T, Nardiello S, Fiorentino F, Galanti B, T cell activation in typhoid fever detected by increased levels of adenosine deaminaseAdvances in Experimental Medicine and Biology 1984 165(Part A):253-56. [Google Scholar]
[30]. Galanti B, Nardiello S, Russo M, Fiorentino F, Increased lymphocyte adenosine deaminase in typhoid feverScandinavian Journal of Infectious Diseases 1981 13:47-50. [Google Scholar]
[31]. Nardiello S, Russo M, Pizzella T, Galanti B, Selectively increased adenosine deaminase levels in T cells during typhoid feverJournal of Clinical and Laboratory Immunology 1984 14:103-05. [Google Scholar]