Among all the forms of periodontitis, aggressive periodontitis has received considerable attention due to its peculiar clinical presentation, with a rapid attachment loss and bone destruction and its occurrence around puberty, with an apparent lack of the local factors, in patients with a reasonably good oral hygiene [1]. A variety of factors such as microbial, environmental, genetic and behavioural factors and systemic diseases have been suggested to influence the risk of aggressive periodontitis [2,3]. It can be subdivided into localized and generalized forms [4]. The most common pathogens which are associated with aggressive periodontitis are Aggregatibacter actinomycetemcomitans and Porphyromonas gingivalis. The list also includes Prevotella intermedia, Tannerella forsythia, Campylobacter rectus, Fusobacterium species and Spirochetes [5].
Various modalities of treatment approaches have been implicated for aggressive periodontitis, among which mechanical therapy Sectionplays an important role. Since the major periodontal pathogens may escape from the treatment due to their ability to invade the periodontal tissues or because they reside at sites which are inaccessible to the periodontal instruments, the mechanical therapy needs to be combined with systemic antibiotics and surgery. Antibiotics which are delivered either locally or systemically, are used as a valuable adjunct to the mechanical therapy. Among all the antibiotics, doxycycline has been found to be the most efficacious in the treatment of aggressive periodontitis [6,7].
The overuse, misuse and the widespread prophylactic application of antibiotics have led to the emergence of drug resistant micro-organisms.
The use of antibiotics may also disturb the indigenous microflora of the body, which include the Lactobacilli in the oral cavity, as well as those in the gastrointestinal tract and the vagina [8]. Hence, it is advantageous to avoid the use of antibiotics that are highly active against Lactobacilli.
Lactobacilli play an important role in the maintenance of health by stimulating the natural immunity as well as by contributing to the balance of the microflora, by interacting with the other members of the flora. The application of health-promoting bacteria for therapeutic purposes, is one of the strongest emerging fields. Time has come to shift the paradigm of the treatment from specific bacteria elimination to alteration of the bacterial ecology by using probiotics [9].
Probiotics are live microorganisms, which, when administered in an adequate amount, confer a health benefit on the host [10]. The vast majority of probiotic bacteria belong to the genera, Lactobacilli, Bifidobacterium, Propionibacterium and Streptococcus. The potential benefits of probiotics have been studied in gastrointestinal disorders, gynaecology, and atopic eczema [11]. Probiotics are available as sour cream, yoghurt, powdered milk, buttermilk and frozen desserts. Probiotics have also been introduced in capsules, tablets and lozenges.
Anatomically, the oral cavity is connected to the whole body and due to this, the oral cavity is influenced by and it influences the general health. Since the oral microbiota is as complex as the gastro-intestinal or vaginal microbiota and as dental biofilms are considered to be difficult therapeutic targets, the encouraging effects of probiotics in different fields of healthcare have resulted in the introduction of probiotics for the oral healthcare. Probiotics have been found to be efficacious in the treatment of halitosis, oral candidiasis and tooth decay [11]. Probiotics have also been introduced in the field of periodontal healthcare and they have been found to be efficacious in the treatment of gingivitis and periodontitis [11].
To the best of our knowledge, there are no studies which have reported the effect of probiotics on aggressive periodontitis and also there are no studies which have compared the effects of probiotics and antibiotics. Hence, this study was conducted by using the lozenges of the probiotic-Inersan® (Lactobacillus brevis CD2) alone, a combination of the probiotic with doxycycline and doxycycline alone, to evaluate their effects on the clinical condition as well as on the Lactobacilli and the A. actinomycetemcomitans counts in aggressive periodontitis patients. Inersan® lozenges were used in the present study as this was the only probiotic medication which was available for dental purposes.
METHODS
The Study Population and Its Randomization
The patients for the present study were selected from the outpatients who presented to the Department of Periodontology, J.S.S. Dental College and Hospital, Mysore, Karnataka, India. The recruitment of these patients was carried out from April 2010 to August 2011. The patients were explained the selected procedure in detail. A prior written consent was taken from all the patients, which was based on the Declaration of Helsinki (1964). This study was approved by the Institutional Review Board of J.S.S. Dental College and Hospital.
The patients who satisfied the following criteria were included in the study. By block randomization, a total of thirty patients of both genders (fourteen males and sixteen females; mean age 24.83 ± 4.42 years, age range 14-35 years) were included, who had sites with a probing depth and a loss of the clinical attachment level which were ≥ 5 mm,and with a radiographic evidence of bone loss. The 596patients were excluded if they had debilitating systemic diseases, if they received antibiotics or anti-inflammatory drugs during the six months prior to the study, if they received periodontal therapy during the six months prior to the study, if they had dental caries, psychiatric disorders, lactose intolerance and allergy to doxycycline or probiotics (Inersan®) and if they were pregnant or smokers All the patients were assigned to one of three groups, which pertained to ten patients in each group.
The Sample Size Determination
The prevalence of aggressive periodontitis in geographically diverse populations is estimated to be below 1% [12]. Based on the past records, thirty patients were expected during the study period.
Probiotic and Antibiotic Products
The products which were used in this study included lozenges of the probiotic, Inersan® (VSL Pharmaceuticals, Inc., USA) which contained 108 Colony-Forming Units (CFU) per gram of L. brevis and tablets of the antibiotic, doxycycline (100 mg). All the patients were instructed to consume the medications for fourteen days according to their group, as follows: Those in the group A consumed lozenges of the probiotic, Inersan® twice (1-0-1) every day for fourteen days. They were directed to place the lozenges in the oral cavity for a few minutes, allowing them to dissolve. Those in the group B consumed lozenges of the probiotic, Inersan® twice (1-0-1) and a doxycycline tablet once (0-1-0) every day for fourteen days. They were instructed to place the lozenges in the oral cavity for a few minutes, allowing them to dissolve and to consume one doxycycline tablet. Those in the group C consumed a doxycycline tablet once (0-1-0) every day for fourteen days.
The Clinical Procedure
The clinical and microbiological parameters were recorded on day 0, at 2 weeks and at 2 months. At day 0, before recording the clinical parameters, 0.5 ml of unstimulated saliva was collected into sterile plastic tubes and it was stored at 4 °C till the time of its microbiological evaluation. The clinical parameters which were recorded were the plaque index (Loe 1967) [13], the gingival index (Loe 1967) [13], the Probing Pocket Depth (PPD) and the Clinical Attachment Level (CAL). After that, Scaling and Root Planing (SRP) was performed on day 0. The SRP was performed by using ultrasonic (Cavitron® - Bobcat Pro, Dentsply) and hand instruments (Gracey Curettes-Hufriedy®). After the SRP, the patients were instructed to practise regular oral hygiene habits. Tooth brushing was done two times per day by using a toothbrush and a toothpaste which they had been using before, and none of the teeth received any other treatment during this study. During the study, the participants followed their usual dietary habits, but they were instructed to refrain from using any other commercial mouth rinses. Two weeks after the SRP, the patients were recalled for the saliva sample collection and for the evaluation of the clinical parameters. On the same day, medications were given to the patients for fourteen days according to the groups which they belonged to. The patients were then recalled at two months for the saliva sample collection and for the evaluation of the clinical parameters.
The Microbiological Procedure
The collected saliva samples were taken for a microbiological evaluation by a culture method, to detect the presence of Lactobacilli and A. actinomycetemcomitans. The saliva samples were serially diluted with peptone water (Oxoid, Unipath, Basingstoke, UK) and sta100 μl of appropriate dilutions were plated to analyze the growth of the Lactobacilli and A. actinomycetemcomitans. de Man-Rogosa-Sharpe (MRS) agar was used for the isolation of Lactobacilli [14]. Tryptone soya agar which was supplemented with yeast extract (0.1%), horse serum (10%), bacitracin (75μg/ml) and vancomycin (5μg/ml) (TSBV), was used for the isolation of A. actinomycetemcomitans [15]. The plates were incubated in a microaerobic atmosphere (10% CO2) for 72 hours at 370C. After 3 days of incubation, the Lactobacilli and the A. actinomycetemcomitans counts were estimated by standard methods [16]. The semi-quantitative colony count was expressed in colony forming units/millilitre (CFU/ml).
STATISTICAL ANALYSIS
The statistical analysis for all the parameters was performed by using descriptive statistics, one way ANOVA, repeated measure ANOVA and the paired sample ‘t’ test. Univariate descriptive statistics were used to calculate the standardized values. The difference between the means was analyzed by using the one way ANOVA procedure. The repeated measure of ANOVA was used to evaluate the differences in time within the groups. All the analyses were performed by using a statistical software program, SPSS version 17.
RESULTS
Characteristics of the Subjects in all the Three Groups on Day 0
No statistically significant difference was found for any clinical or microbiological parameter in all the three groups on day 0 (p > 0.05) [Table/Fig-1].
Comparison of clinical and microbiological parameters at day 0 between all the three groups
| Groups | Mean | Std. Deviation | F Value | Significance (p) |
|---|
| Plaque Index | A | 0.61 | 0.18 | | |
| B | 0.95 | 0.43 | 1.58 | 0.23 |
| C | 0.80 | 0.30 | | |
| Gingival Index | A | 2.00 | 0.00 | | |
| B | 2.03 | 0.05 | 2.50 | 0.11 |
| C | 2.00 | 0.00 | | |
| Probing Pocket Depth (mm) | A | 3.79 | 0.41 | | |
| B | 3.75 | 0.72 | 0.01 | 0.98 |
| C | 3.75 | 0.31 | | |
| Clinical Attachment Level (mm) | A | 3.27 | 0.40 | | |
| B | 3.50 | 0.95 | 0.39 | 0.68 |
| C | 3.74 | 1.20 | | |
| Lactobacilli | A | 1.07 × 105 | 1.76 × 105 | | |
| (CFU/ml) | B | 1.70 × 105 | 2.86 × 105 | 2.28 | 0.13 |
| C | 5.99 × 105 | 6.72 × 105 | | |
| A. actinomycetem | A | 3.33 × 106 | 4.51 × 106 | | |
| comitans | B | 1.85 × 106 | 3.03 × 106 | 1.38 | 0.28 |
| (CFU/ml) | C | 3.14 × 105 | 2.95 × 105 |
Descriptive Statistics with one way ANOVA
* Statistically significant p < 0.05
Changes in the Clinical Parameters
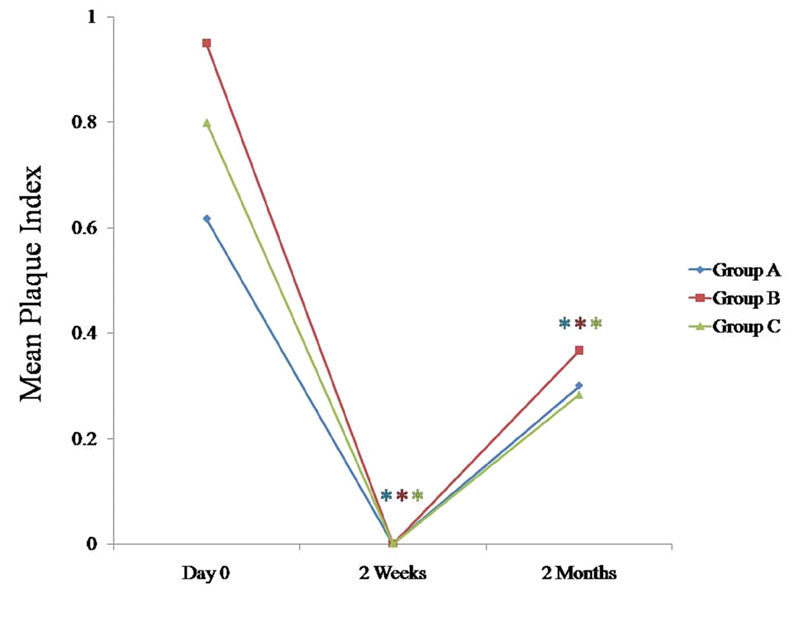
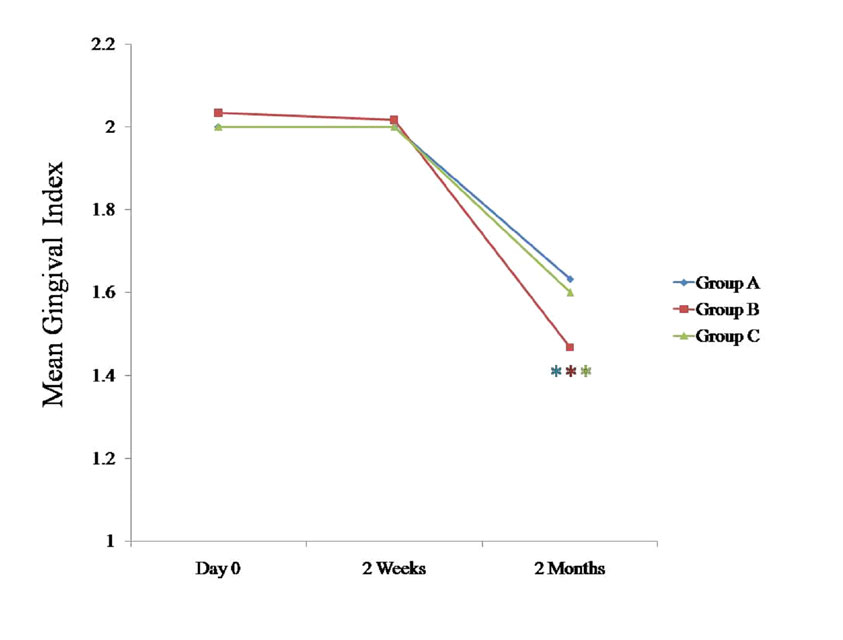
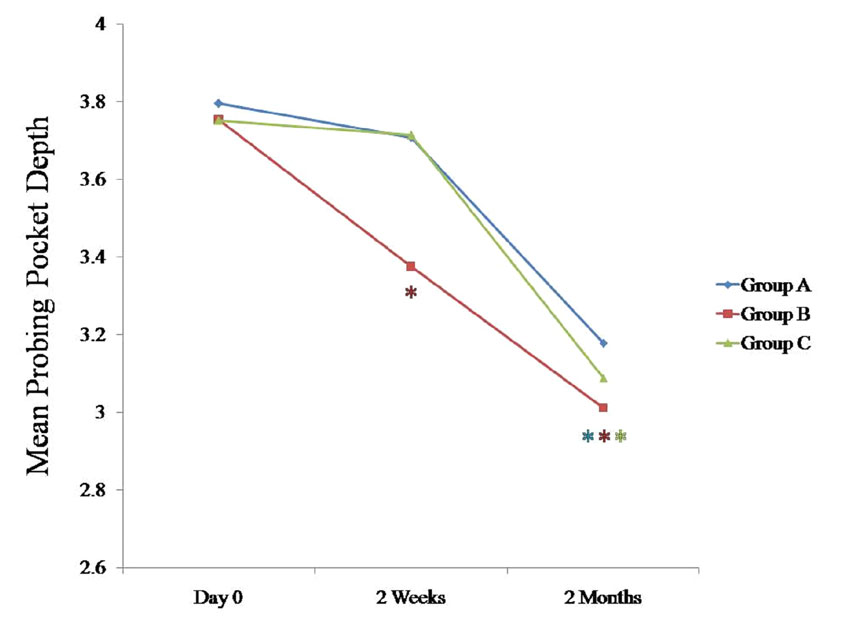
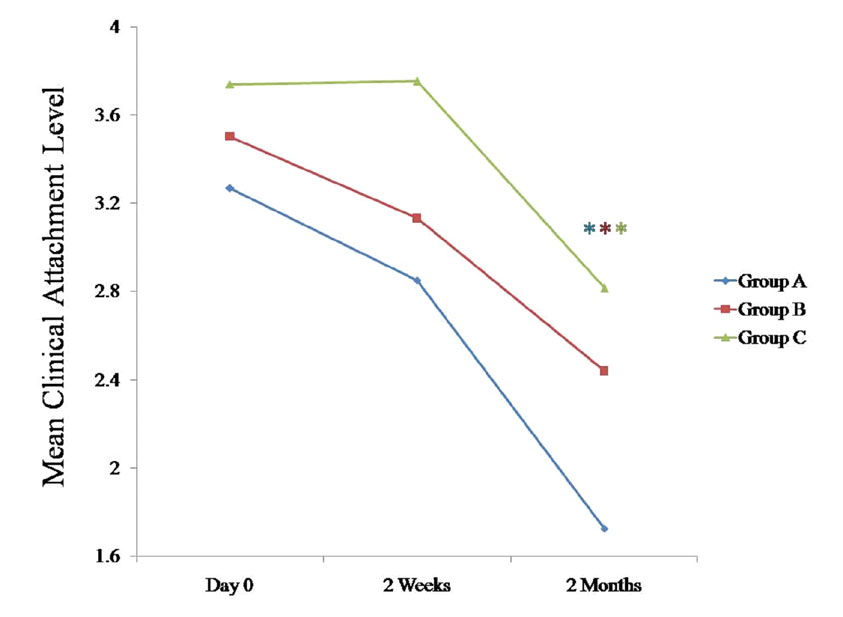
We compared the changes in the clinical parameters between all the three groups at various intervals [Table/Fig-1], [Table/Fig-2], [Table/Fig-3], [Table/Fig-4]; [Table/Fig-5], [Table/Fig-6], [Table/Fig-7], 597 [Table/Fig-8]. The plaque index, the gingival index, the PPD and the CAL tended to decrease significantly (p < 0.05) in all the three groups at 2 months when they were compared with the findings on day 0 [Table/Fig-5], [Table/Fig-6], [Table/Fig-7], [Table/Fig-8]. When these data were calculated with repeated measure ANOVA, a statistically significant change (p < 0.05) which was irrespective of the groups was observed from day 0 to 2 months. When the changes with respect to the three groups were verified, a statistically non-significant change was observed (p > 0.05) [Table/Fig-4].
Comparison of clinical and microbiological parameters at 2 weeks between all the three groups
| Groups | Mean | Std. Deviation | F Value | Significance (p) |
|---|
| Plaque Index | A | 0.00 | 0.00 | | |
| B | 0.00 | 0.00 | - | - |
| C | 0.00 | 0.00 | | |
| Gingival Index | A | 2.00 | 0.00 | | |
| B | 2.01 | 0.04 | 1.00 | 0.39 |
| C | 3.70 | 0.68 | | |
| Probing Pocket Depth (mm) | A | 3.37 | 0.77 | | |
| B | 3.71 | 0.29 | 0.58 | 0.57 |
| C | 3.75 | 0.31 | | |
| Clinical Attachment Level (mm) | A | 2.85 | 0.84 | | |
| B | 3.13 | 0.87 | 1.19 | 0.33 |
| C | 3.75 | 1.31 | | |
| Lactobacilli | A | 8.51 × 105 | 1.51 × 105 | | |
| (CFU/ml) | B | 4.38 × 105 | 4.57 × 105 | 2.72 | 0.09 |
| C | 2.57 × 106 | 2.43 × 106 | | |
| A. actinomycetem | A | 4.68 × 105 | 7.13 × 105 | | |
| comitans | B | 1.05 × 105 | 1.17 × 105 | 1.24 | 0.31 |
| (CFU/ml) | C | 1.56 × 105 | 1.92 × 105 | | |
Descriptive Statistics with one way ANOVA
* Statistically significant p < 0.05
Comparison of clinical and microbiological parameters at 2 months between all the three groups
| Groups | Mean | Std. Deviation | F Value | Significance (p) |
|---|
| Plaque Index | A | 0.30 | 0.12 | | |
| B | 0.36 | 0.05 | 1.43 | 1.43 |
| C | 0.28 | 0.07 | | |
| Gingival Index | A | 1.63 | 0.10 | | |
| B | 1.46 | 0.05 | 1.72 | 0.21 |
| C | 1.60 | 0.26 | | |
| Probing Pocket Depth (mm) | A | 3.17 | 0.19 | | |
| B | 3.01 | 0.75 | 0.14 | 0.86 |
| C | 3.08 | 0.49 | | |
| Clinical Attachment Level (mm) | A | 1.72 | 0.55 | | |
| B | 2.43 | 1.13 | 1.11 | 0.35 |
| C | 2.81 | 1.83 | | |
| Lactobacilli | A | 6.83 × 106 | 6.91 × 106 | | |
| (CFU/ml) | B | 3.75 × 106 | 8.45 × 106 | 1.76 | 0.20 |
| C | 6.03 × 103 | 7.37 × 103 | | |
| A. actinomycetem | A | 1.00 × 104 | 1.43 × 104 | | |
| comitans | B | 1.18 × 103 | 1.29 × 103 | 2.13 | 0.15 |
| (CFU/ml) | C | 1.06 × 105 | 1.69 × 105 |
Descriptive Statistics with one way ANOVA
* Statistically significant p < 0.05
Comparison of clinical and microbiological parameters between all the three groups from day 0 to 2 months
| | F Value | Significance (p) |
|---|
| Plaque Index | Change * | 75.76 | 0.00 |
| Change between groups | 1.31 | 0.28 |
| Gingival Index | Change * | 127.95 | 0.00 |
| Change between groups | 2.30 | 0.08 |
| Probing Pocket Depth | Change * | 22.95 | 0.00 |
| Change between groups | 0.57 | 0.68 |
| Clinical Attachment Level | Change * | 50.84 | 0.00 |
| Change between groups | 1.54 | 0.21 |
| Lactobacilli | Change * | 4.01 | 0.02 |
| Change between groups * | 2.70 | 0.04 |
| A. actinomycetemcomitans | Change * | 5.54 | 0.00 |
| Change between groups | 1.38 | 0.26 |
Descriptive Statistics with repeated measure ANOVA
* Statistically significant p < 0.05
Comparison of mean plaque index at various time intervals between all the three groups
* Statistically significant p < 0.05
Comparison of mean gingival index at various time intervals between all the three groups
* Statistically significant p < 0.05
Comparison of mean probing pocket depth (mm) at various time intervals between all the three groups
* Statistically significant p < 0.05
Comparison of mean clinical attachment level (mm) at various time intervals between all the three groups
* Statistically significant p < 0.05
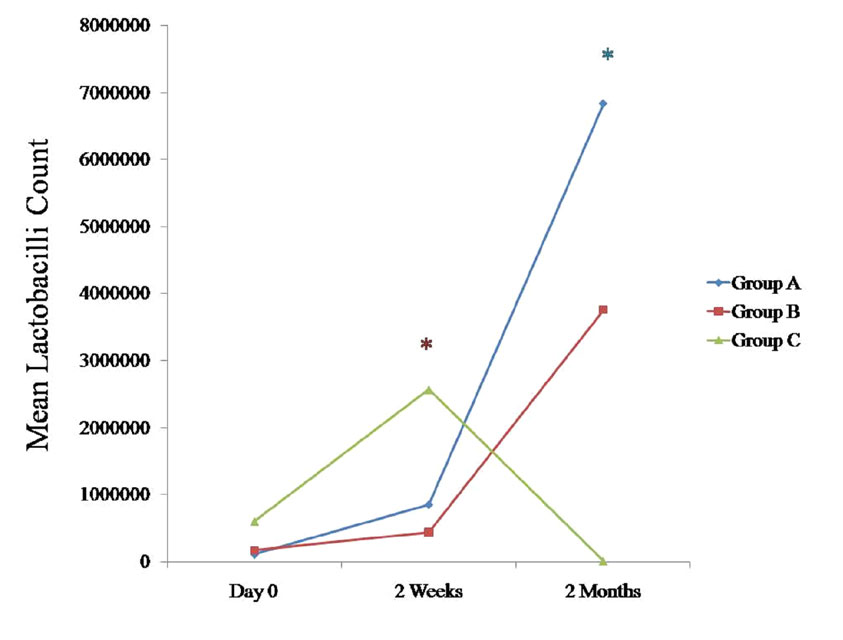
Changes in the Lactobacilli Counts in Saliva
We compared the changes in the Lactobacilli counts in saliva between all the three groups at various intervals [Table/Fig-1], [Table/Fig-2], [Table/Fig-3], [Table/Fig-4], [Table/Fig-9]. The Lactobacilli counts in the saliva tended to increase for both the groups A and B and they decreased for group C at 2 months as compared to the counts on day 0. They were found to be statistically significant for group A (p < 0.05) [Table/Fig-9]. When these data were calculated with repeated measure ANOVA, a statistically significant change (p < 0.05) which was irrespective of the groups was observed from day 0 to 2 months. When the changes with respect to the three groups were verified, a statistically significant change was observed (p < 0.05) [Table/Fig-4].
Comparison of mean Lactobacilli count (CFU/ml) at various time intervals between all the three groups
* Statistically significant p < 0.05
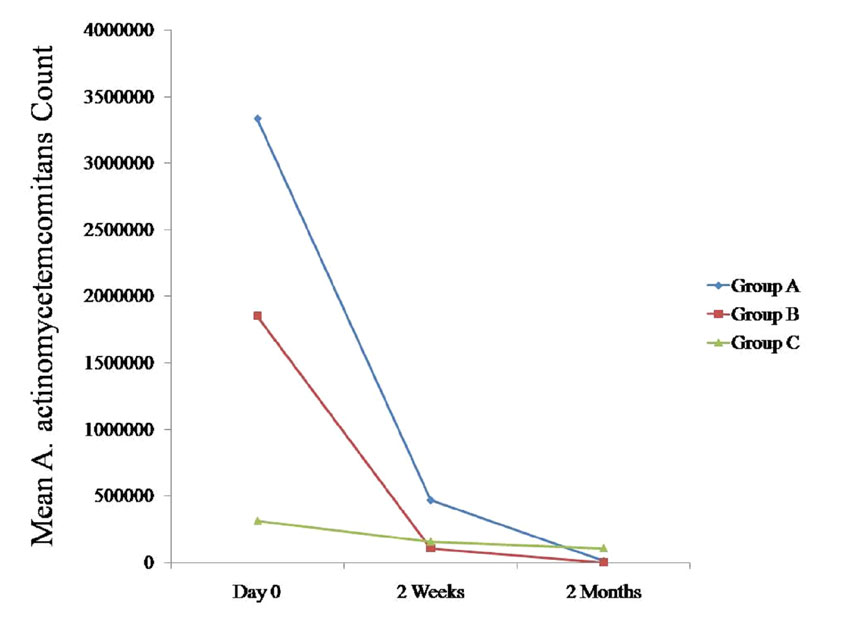
Changes in the A. Actinomycetemcomitans Counts in Saliva
We compared the changes in the A. actinomycetemcomitans counts in saliva between all the three groups at various intervals [Table/Fig-1],[Table/Fig-2],[Table/Fig-3],[Table/Fig-4],[Table/Fig-10]. The A. actinomycetemcomitans counts tended to decrease in all the three groups at 2 months as compared to the counts on day 0, which was statistically non-significant (p > 0.05) [Table/Fig-10]. When these data were calculated with repeated measure ANOVA, a statistically significant change (p < 0.05) which was irrespective of the groups was observed from day 0 to 2 months. When the changes with respect to the three groups were verified, a statistically non-significant change was observed (p > 0.05) [Table/Fig-4].
Comparison of mean A. actinomycetemcomitans count (CFU/ml) at various time intervals between all the three groups
* Statistically significant p < 0.05
DISCUSSION
This was a pilot, randomized, open trial which was done to evaluate and compare the effects of the probiotic, (Inersan®) alone, a combination of the probiotic with doxycycline and doxycycline alone, in the treatment of aggressive periodontitis. The clinical parameters (the plaque index, the gingival index, the PPD and the CAL) and the microbiological parameters (Lactobacilli and A. actinomycetemcomitans) were recorded on day 0, at 2 weeks and at 2 months.
Our findings indicated that there was a statistically significant reduction in the plaque index in all three groups at 2 months as compared to the findings on day 0 (p < 0.05). This was in accordance with the findings of five other studies which had reported a reduction in the amount of plaque index as compared to the baseline value for the probiotic group [17–21].
The gingival index tended to decrease in all three groups at 2 months as compared to the findings on day 0, which was statistically significant (p < 0.05). Five other studies also detected statistically significant decreases in the gingival scores as compared to the baseline values for the probiotic group [17,19–22].
The PPD tended to decrease in all three groups at 2 months as compared to the findings on day 0, which was statistically significant (p < 0.05). Of the three human studies that reported a decrease in the PPD, [21,23] only two studies could detect a statistically significant decrease in the PPD as compared to the baseline values for the probiotic group [20,21].
The CAL tended to decrease significantly in all three groups at 2 months as compared to the findings on day 0 (p < 0.05). Only one study had reported a statistically significant decrease in the CAL as compared to the baseline value for the probiotic group [21].
We found that the SRP resulted in an increase in the Lactobacilli counts in all the three groups. There was an increase in the Lactobacilli counts in Group A and Group B while there was a decrease in the Lactobacilli counts in Group C at 2 months, which also supported the findings of a previous study, which had determined the antibiotic susceptibility pattern of the Lactobacilli strains to twenty-nine antibiotics, and had found them to be susceptible to a wide variety of antibiotics, which included amoxicillin, tetracycline and clindamycin [8].
We found that the A. actinomycetemcomitans counts tended to decrease in all three groups at 2 months as compared to the findings on day 0, which was statistically non-significant (p > 0.05). Of the four human studies that had reported a reduction in the A. actinomycetemcomitans counts, [9,20,22,23] only two studies could detect a statistically significant decrease in the A. actinomycetemcomitans counts as compared to the baseline value for the probiotic group [21,24].
A study had already reported on the potential effects of the L. brevis extracts containing lozenges in the periodontitis patients. Their findings had shown that all the inflammatory-associated factors had been drastically reduced in the periodontal disease patients after the L. brevis-based treatment. The anti-inflammatory effects of L. brevis could be attributed to the presence of arginine deiminase which had prevented the nitric oxide generation [19]. This was the mechanism by which the probiotic (Inersan®) lozenges acted. The probiotics also produce different antimicrobial components which include organic acids, hydrogen peroxide, low-molecular weight antimicrobial substances, bacteriocins, and adhesion inhibitors [25].
In the present study, it was found that the administration of probiotic alone, a combination of the probiotic with doxycycline and doxycycline alone, had resulted in decreased clinical parameters and A. actinomycetemcomitans counts. The Lactobacilli counts had tended to increase in the probiotic alone group and in the group with a combination of the probiotic with doxycycline and a decrease in the doxycycline alone group. Even though doxycycline was found to effectively reduce the A. actinomycetemcomitans counts, it had a negative impact on the Lactobacilli counts. Doxycycline may also lead to the development of drug resistance.
Probiotics have a future in the treatment of aggressive periodontitis, as antibiotics are prescribed most of the time. These antibiotics can lead to the emergence of drug resistant micro-organisms and they can also disturb the beneficial microflora of the body. Thus, as an alternative to antibiotics, probiotics can be used, as they repopulate the beneficial microflora and reduce the pathogenic bacteria. Hence, a treatment with probiotics could be an ideal alternative in the management of aggressive periodontitis.