Dementia is a clinical syndrome whose main element is memory impairment. More than 75% of the dementia cases are caused by Alzheimer’s disease. Alzheimer’s disease, on the other hand, is a neuropathological entity that is characterized by a protracted preclinical phase, which is followed by the onset of a slowly progressive dementia. About 60% of the demented patients manifest the typical pathological findings of Alzheimer’s disease—amyloid deposits and neurofibrillary tangles—, without any other abnormalities in the brain, while a further 15% have these findings accompanied by brain damage which is of vascular origin. Dementia which is caused by vascular lesions alone, accounts for fewer than 15% of the total dementia cases. Lewy-body dementia which is usually accompanied by Parkinsonism and marked fluctuations of consciousness and Frontotemporal Lobar Degeneration (FTLD), each account for about 5% of the cases of dementia. According to epidemiological data, 5% of the dementia cases occur secondary to another disease ; the causes in this category include endocrine disorders such as hypothyroidism and hyperparathyroidism [1, 2].
Age remains the single most important risk factor for developing dementia. Epidemiological studies have shown that dementia affects one in twelve persons who are over the age of 65 years and one in three persons who are over the age of 90 years. Beyond the age of 65 years, the prevalence of dementia doubles for every 5 years of life; early-onset dementia is encountered, especially in those who are susceptible or in those who harbour predisposing factors, or in those who have developed any type of central affections [3, 4].
Considering the progressive aging of the general population, the increasing prevalence of early-onset dementia, the increasing percentage of literacy and the weak medical knowledge on the symptoms of dementia, the screening tests for susceptible persons assume significance. A neuropsychometric assessment seems to be the best method for screening individuals. However, the lack of standardization of the screening tools has to be recognized as a major issue in the estimation of the true burden. A standardization might not be readily achieved because of the diversity of the language, culture, and the levels of literacy. In certain communities, more than 80% of the elderly people do not read or write. The Mini Mental State Examination (MMSE) has been translated into many languages and its use as an initial screening tool was settled [5, 6].
The Clock Drawing Test (CDT) has been extolled as an inexpensive, fast, “non-threatening” and an easily administered measure of the cognitive function, especially in the elderly. CDT is a multifaceted and a multidimensional measure test which is thought to test the visuoconstructive and the visuospatial skills, the symbolic and the graphomotor representation, the auditory language skills, the hemiattention, the semantic memory, the conceptual abilities, and the executive function, which includes organization, planning, and parallel processing [7, 8].
The current prospective study was aimed at evaluating the discriminative abilities of MMSE and CDT in the differentiation of the demented patients from the controls and the differentiation between the types of dementia.
PATIENTS AND METHODS
This prospective study was conducted in the Neurology Department, King Fahd Hospital, Hufof , Saudi Arabia, from June 2007 to June 2011. All the patients with varied types and severities of dementia, who attended the Neurology Outpatients Clinic were included in the study. The patients were diagnosed with regards to the type and the severity of dementia by using the Clinical Dementia Rating (CDR) scale [9]. All the patients underwent routine laboratory investigations, which included a complete blood count, random blood glucose levels, renal function and liver function tests and the thyroid hormone profile. Other investigations like CT or MR imaging were done as per the need.
All the patients underwent evaluation of the following demographic and social variables: age assessment which included a 5-year age grouping, the gender and marital statuses, the level of education (illiterate or educated), occupation and the living arrangement. The smoking status was categorized as “nonsmoker,” “ex-smoker,” and “current smoker.” The general health status was evaluated with an emphasis on the past and present history of chronic diseases, especially cardiac diseases, cerebrovascular diseases, Parkinson’s disease, Diabetes mellitus and cancer.
All the patients were assessed for neuropsychatric manifestations which included the presence of the signs of extra-pyramidal, pyramidal and cerebellar affections. The presence of paranoid or other delusional ideations, hallucinations, psychomotor activity disturbances, aggressiveness or affective disturbances, was assessed. All the clinical and radiological data were evaluated for the categorization of the dementia patients according to the type of dementia.
All the patients completed a Mini Mental State Examination; the total and the sub-scores were calculated and the final score was determined according to the guidelines for the standardized MMSE [10, 11]. The Clock-Drawing test (CDT): to minimize the effect of education, a simple scoring system [12] was used. All the patients were allowed to see a large-sized wall watch prior to the drawing and to turn away from it to start drawing. The following three items were evaluated: A correctly drawn clock shape, all the numbers being in the correct position and the hands of the clock being set to the correct time. A score of 1was assigned for each of these items; thus, the score could range from 0 (all items incorrect) to 3 (all items correct). The presence of bizarre drawings was scored as 0 and if they were different, it was scored as 1. Therefore, the final possible scores ranged between 0 (the worst) and 4 (the best).
The study also included 30 control subjects for the evaluation of the results of MMSE and CDT. Age-matched controls were selected from the patients who were admitted to the General Surgery Department for minor surgical procedures, with the CDR rate ranging between 0 and 0.5 and they were neurologically free.
STATISTICAL ANALYSIS
The obtained data were presented as mean±SD and ranges. The results were analyzed by using the Wilcoxon ranked test for the unrelated data (Z test) and the Chi-square test (X2 test). The statistical analysis was conducted by using the SPSS (version 15, 2006) for the Windows Statistical Package. A P value of <0.05 was considered as statistically significant.
RESULTS
The study included 197 patients; 115 males (58.4%) and 82 females (41.6%) with a mean age of 68.6±7.6; 43-79 years. Fifty-four patients (27.6%) were free of medical co-morbidities, while 142 patients (82.4%) had additional medical co-morbidities. Diabetes mellitus was the most frequent among these co-morbidities. Sixty-three patients (32%) had good-excellent general health and 95 patients (48.2%) had good general health, while 39 patients (19.8%) had poor-fair general health. The patients’ enrollment criteria are shown in [Table/Fig-1].
Patients enrollment data
Data are presented as numbers & mean±SD; percentages & ranges are in parenthesis
| Strata | Number (%) | Mean±SD |
|---|
| Age (years) | <60 | 23 (11.7%) | 54.2±4.3 (43-59) |
| 60-65 | 38 (19.3%) | 62.3±1.7 (60-65) |
| >65-70 | 45 (22.8%) | 68±1.3 (66-70) |
| >70-75 | 46 (23.4%) | 73.3±1.2 (71-75) |
| >75-80 | 45 (22.8%) | 77.4±1.1 (76-79) |
| Total | 68.7±7.7 (55-79) |
| Gender | Males | 115 (58.4%) | |
| Females | 82 (41.6%) | |
| Marital Status | Married | 97 (49.2%) | |
| Divorced | 15 (7.6%) | |
| Widow | 66 (33.5%) | |
| Single | 19 (9.7%) | |
| Educational level | Illiterate | 178(90.4%) | |
| Educated | 15 (9.6%) | |
| Smoking | Never | 154 (31.8%) | |
| Stopped | 196 (40.4%) | |
| Still | 135 (27.8%) | |
| Care provision | Living alone with the other partner | 56 (11.5%) | |
| Living with the family | 178 (36.7%) | |
| Living care provider | 134 (27.6%) | |
| Living in elderly home | 65 (13.4%) | |
| Living alone with family member | 52 (10.7%) | |
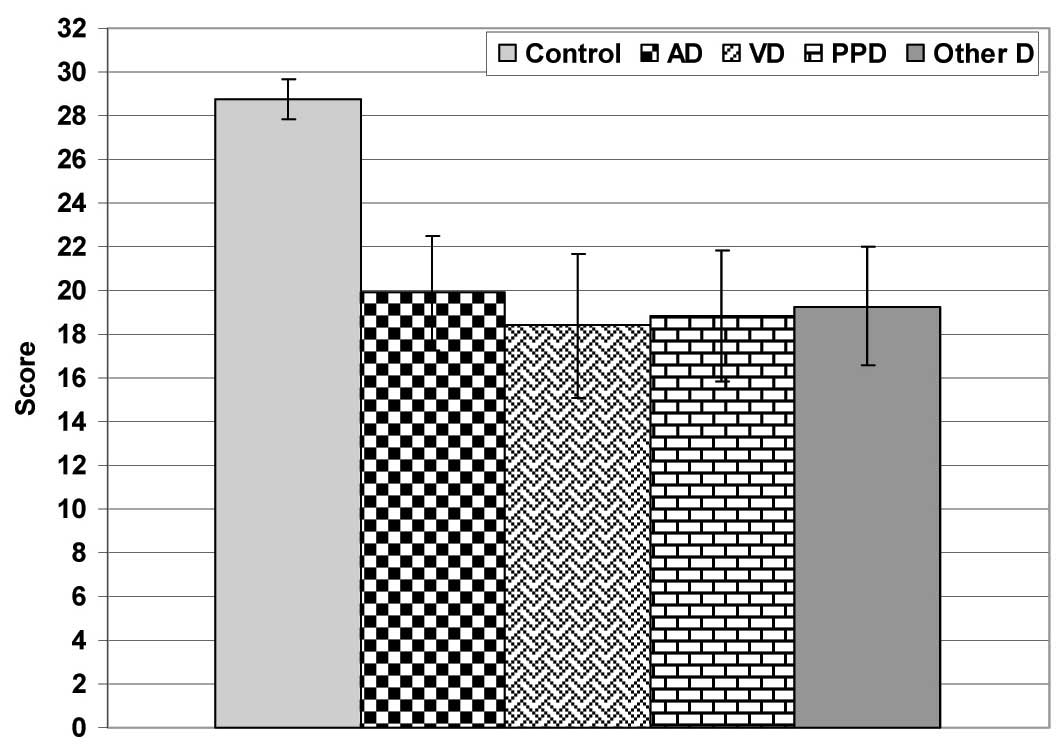
The clinical and radiological evaluations defined 51 patients (25.9%) as having AD, 37 patients (18.8%) as having VD, 23 patients (11.7%) as having PDD and 86 patients (43.6%) as having other variants of dementia. The total MMSE score of the enrolled patients (19.2±2.8) showed significantly (p<0.05) lower scores as compared to those of the control subjects (28.8±0.9). Differentially, according to the type of dementia, the recorded MMSE estimates were significantly lower (p<0.05) as compared to those of the control group, but with a non-significant (p<0.05) difference among the patients of varied diagnoses [Table/Fig-2].
MMES of studied patients categorized according to type of dementia
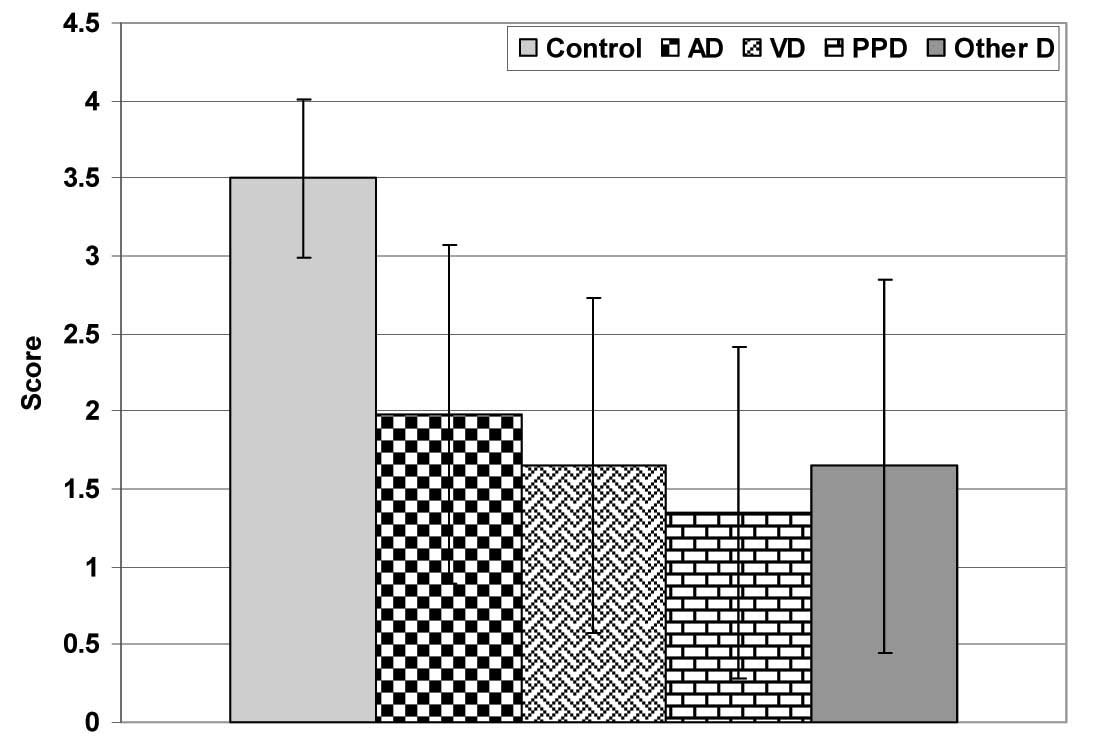
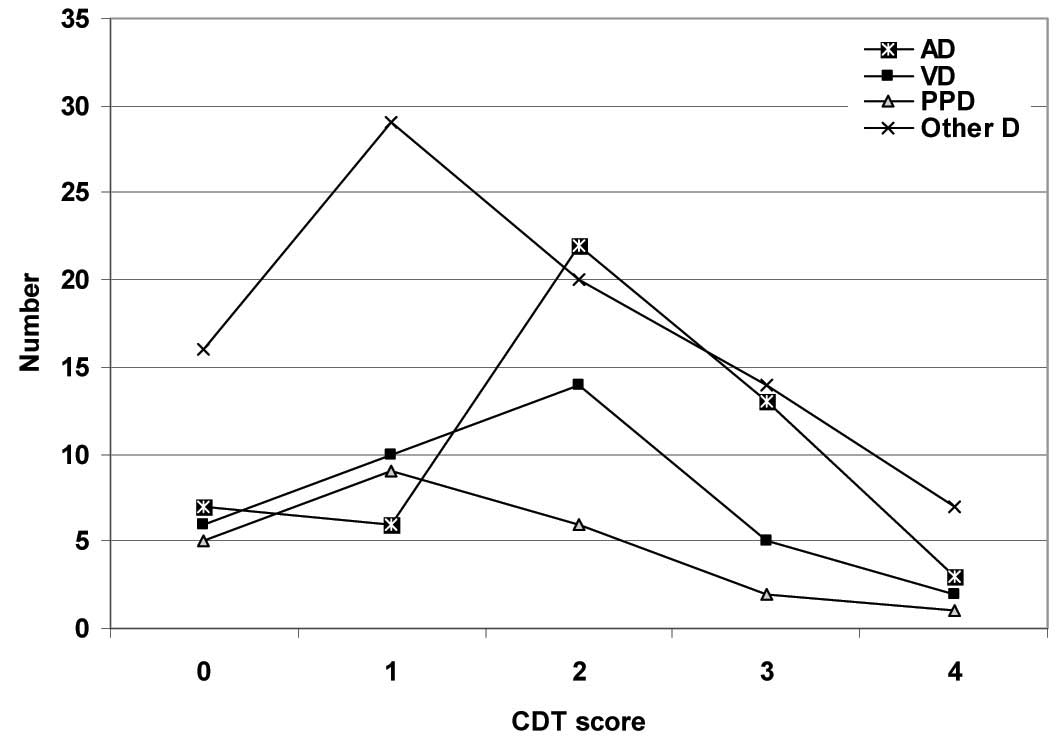
The total CDT scores were significantly (p<0.05) lower in the patients as compared to those in the control subjects, with significantly (p<0.05) lower scores in the PDD group as compared to those in the AD group. The patients who had AD showed non-significantly (p<0.05) higher CDT scores as compared to the patients who had vascular and other types of dementia, with a non-significant (p<0.05) difference between both the latter groups. Also, the PDD group showed non-significantly (p<0.05) lower CDT scores as compared to the patients who had vascular and other types of dementia, [Table/Fig-3]. As regards the patients’ distribution among the CDT scores, the patients who had AD showed a significantly higher frequency of higher scores as compared to those who had VD (X2=3.416, p<0.05), PPD (X2=7.153, p<0.05) and other types of dementia (X2=9.221, p<0.05), with a non-significant (p<0.05) difference among the other groups, but in favour of the VD group [Table/Fig-4 and 5].
CDT scores of studied patients categorized according to type of dementia
Results of MMSE and CDT of studied patients categorized according to type of dementia and compared to control subjects
| Control | AD | VD | PDD | Other types |
|---|
| Number | 30 | 51 (25.9%) | 37 (18.8%) | 23 (11.7%) | 86 (43.6%) |
| MMSE score | 28.8±0.9 | 19.9±2.6* | 18.4±3.3* | 18.9±3* | 19.3±2.7* |
| CDT | 4 | 17 | 3 (5.9%) | 2 (5.4%) | 1 (4.3%) | 7 (8.1%) |
| 3 | 13 | 13 (25.5%) | 5 (13.5%) | 2 (8.7%) | 14 (16.3%) |
| 2 | 0 | 22 (43.1%) | 14 (37.8%) | 6 (26.1%) | 20 (23.3%) |
| 1 | 0 | 6 (11.8%) | 10 (27.1%) | 9 (39.1%) | 29 (33.7%) |
| 0 | 0 | 7 (13.7%) | 6 (16.2%) | 5 (21.8%) | 16 (18.6%) |
| Total score | 3.5±0.5 | 1.98±1.09* | 1.65±1.09* | 1.35±1.07*† | 1.65±1.2* |
Data are presented as numbers & mean±SD; percentages are in parenthesis
*: significant versus control group; †: significant versus AD group
Patient’s distribution according to CDT scores
DISCUSSION
The current study reported a high frequency of co-morbidities among the studied dementia patients, which reached up to about 82% of the studied patients; this finding illustrated a coincidence of the systemic co-morbidities and dementia. This surely intensifies the burden on the caregiver, it consumes much of the resources and it requires a frequent inpatient management. These findings also emphasize the necessity of a proper control of the systemic co-morbidities to allow improvement of the general health. In support of this, only 63 patients (32%) had good-excellent general health and 95 patients (48.2%) had good general health, while 39 patients (19.8%) had poor-fair general health.
Phelan et al., [13] tried to determine whether the dementia onset was associated with higher rates of or different reasons for hospitalization, particularly for Ambulatory Care-Sensitive Conditions
(ACSCs) and they found that the adjusted admission rates were significantly higher in the dementia group and that the adjusted admission rates for all types of ACSCs, which included bacterial pneumonia, congestive heart failure, dehydration, duodenal ulcers, and urinary tract infections, were significantly higher among those with dementia.
One of the interesting findings of the current study was the complaint of fatigue and the easily fatigability of the dementia patients who had Parkinsonism, as compared to the other dementia patients who did not make such a complaint. This could be attributed to the inherent character of Parkinson’s disease and it may not be related to dementia itself. Friedman et al., [14] reported that the non-motor symptoms of Parkinson’s disease have become increasingly recognized as central to the disease and that they include somatic symptoms such as pain and autonomic dysfunction and behavioural problems such as dementia, depression, fatigue, sleep disorders and psychosis. They concluded that fatigue was a common and severe problem in Parkinson’s disease.
In our study, the recorded MMSE estimates were significantly lower among the dementia patients compared to the control group, but with a non-significant difference among the patients of the varied dementia types. These data were comparable with those of the study of Oh et al., [15] who had conducted cognitive screening by using MMSE, with repeated evaluations at 6-months, 1 year, and 2 years after the initial baseline assessment and they had found no difference between the three dementia subtypes; AD, VD and PDD with respect to the baseline MMSE scores.
However, the use of MMSE to screen for dementia may be inappropriate because of the low education level of many patients, and their non-validation in this population [16]. Low education leads to a false positive screening of dementia. A minimum of Grade 8 reading is required for an effective employment of the MMSE [17].
We sought to enhance the effectiveness of the MMSE by further applying the CDT. In the context of the validity of both the tests for the defined target, De Guise et al., [18] compared the performances of the patients with mild, moderate, and severe traumatic brain injuries on the CDT and MMSE and reported that the CDT and MMSE, in combination, had the potential for the prediction of the outcome in the traumatic brain injury population.
It was found that the total CDT scores were significantly lower in our patients as compared to those in the control subjects, with significantly higher scores in the AD group as compared to the PDD group. Non-significantly higher CDT scores as compared to the patients had vascular and other types of dementia.
These data indicated the ability of the CDT to differentiate the patients who had AD from those who had other types of dementia. The combination of the MMSE and the CDT showed a high sensitivity and specificity in a sample of 129 probably dementia patients [19]. Its performance was unaffected by education or language [20]. Aprahamain et al., [21] discriminated illiterate elderly patients with and without Alzheimer’s Disease (AD) in a clinical sample by using varied tests and reported that the best specificity was observed with the combination of the MMSE and the CDT (89%). In a similar vein, Umidi et al., [22] documented that during the screening for a cognitive decline, the administration of both the CDT and the MMSE can be useful for identifying the subjects with a possible Minimal Cognitive Impairment (MCI).
Moreover, Millan et al., [23] reported that the Mini-Cog (The CDT and an extraction of the 3-item recall of the MMSE were used to constitute the Mini-Cog algorithm) showed a significantly higher discriminatory power (86.8%) than the MMSE (72.6% at a cut-off = 24 and 79.2% at = 25, respectively) and the CDT (78.1%) and that it did not perform worse than the algorithm MMSE and/or the CDT (each p > 0.05). The specificity of the Mini-Cog (100.0%) was similar to that of the MMSE (100.0% for both cut-offs) and the CDT (96.9%) (p = 0.154).
It could be concluded that a combined application of both the MMSE and the CDT could identify the persons with a cognitive affection and that this may be a useful tool in the diagnosis of the non Alzheimer’s type of dementia. However, our study was limited only to one facility, which potentially limited the generalization of the current findings. The further studies should utilize population –based samples.
Data are presented as numbers & mean±SD; percentages are in parenthesis
*: significant versus control group; †: significant versus AD group