Platelets are formed and released into the bloodstream by precursor cells called megakaryocytes (MK) that are derived from haematopoietic stem cells (HSCs), which evolve from the multipotential haemangioblast. Mature MKs give rise to circulating platelets by the acquisition of the cytoplasmic structural and functional characteristics necessary for platelet action [1,2], reaching cell sizes <50-100 microns in diameter and ploidy ranging up to 128 N [3,4]. Endoreduplication (polyploidisation) and expansion of cytoplasmic mass are the hallmarks of MK maturation [5]. The production of platelets by megakaryocytes requires an intricate series of remodeling events that result in the release of thousands of platelets from a single megakaryocyte. Abnormalities in this process can result in clinically significant disorders. A diversity of factors can contribute to anomalous platelet counts; one of these is inappropriate platelet production. Thrombocytopenia (platelet counts less than 150,000/μl) can lead to inadequate clot formation and increased risk of bleeding [6]. Thrombocytopenia is commonly encountered in various hematological disorders including myelodysplastic syndromes (MDS) as well as various non-myelodysplastic hematological conditions [7].
Various studies have highlighted the dysplastic morphology of megakaryocytes in thrombocytopenia associated with myelodysplastic syndromes (MDS). Myelodysplastic syndromes (MDS) are a heterogeneous group of clonal hematopoietic stem cell disorders characterized by bone marrow (BM) failure and increased risk of transformation to acute myeloid leukemia (AML) [8]. Megakaryocytic alterations have also been recorded in some bone marrow aspiration (BMA) series in non-myelodysplastic conditions.
The present study was undertaken to calculate the prevalence of various conditions associated with thrombocytopenia and to record the megakaryocytic alterations in various cases of thrombocytopenia; taking in account findings noted in both BMA as well as biopsy (BMB). Apart from this by means of statistical analysis it was tried to analyze whether a significant difference existed in megakaryocytic alteration noted in MDS versus non- MDS conditions.
MATERIALS AND METHODS
A prospective series of 60 bone marrow aspirations along with concomitant bone marrow biopsies was conducted in a tertiary care centre catering to both urban as well as rural population in north India. All the cases of thrombocytopenia which were diagnosed on hematology analyzer (platelet count < 1, 50,000); confirmed subsequently by peripheral smears were taken up for the study. Both BMA and BMB were done from posterior superior iliac spine taking care of all the aseptic measures. Smears were drawn and air dried from the material aspirated and stained by May Grunwald Giemsa (MGG). The biopsy core obtained was fixed with 10 % buffered formaline overnight and then kept for decalcification for a period of 48 to 72 hours depending upon the hardness of the biopsy in 15 % EDTA solution. This was afterwards taken up for processing; 2-3 micron sections were cut and stained by hematoxylin and eosin and other special stains wherever deemed necessary.
Both the BMA and BMB slides were reviewed by 3 haematopathologists in a double blinded way. Findings concurring 2 out of 3 were taken as final and recorded in a systematic table. The clinical details, complete blood counts, and other relevant laboratory investigations were also obtained.
In the present study for scoring purposes the number and morphological
changes were pre-defined before start of the study. The cases were defined according to the work done by Muhury M et al., [7] and broadly the cases were tabulated accordingly. The number of the megakaryocytes was considered as normal (one megakaryocyte per one to three low-power fields), increased (more than two megakaryocytes per low-power field) or decreased (one 474megakaryocyte per five to ten low-power fields) [9].
The morphological changes of megakaryocytes that were studied included- nuclear segmentation, presence of immature forms, dysplastic forms, micromegakaryocytes, emperipolesis, platelet budding, cytoplasmic vacuolization, bare megakaryocytic nuclei and hypogranular forms. The presence of abnormal megakaryocytes which included the micromegakaryocytes, dysplastic forms, megakaryocytes with separated lobes and hypogranular forms were considered as dysmegakaryocytopoiesis [Table/Fig-1, 2].
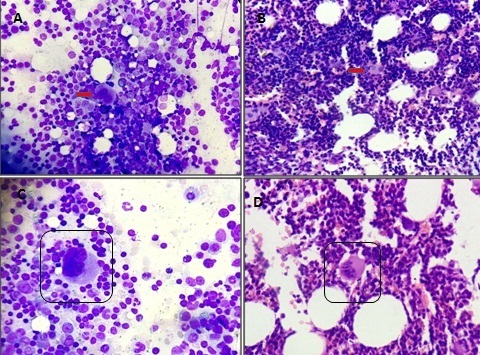
A: Collection of hypolobated megakaryocytes in bone marrow aspirate. (MGG 400 X) B: Collection of hypolobated megakaryocytes in bone marrow biopsy section. (H& E 100X) C: Highpower view of a hypolobated immature megakaryocyte in aspirate. (MGG 1000 X) D: Highpower view of a hypolobated immature megakaryocyte in biopsy. (H & E 400 X)
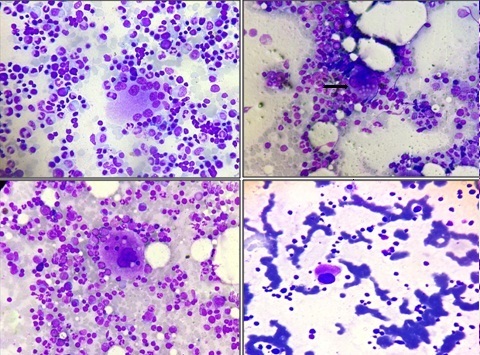
A: Multi nucleated dysplastic form of megakaryocyte in bone marrow aspirate. (MGG 400 X) B: Cytoplasmic vacuolization of megakaryocytes noted in infection associated thrombocytopenia (IAT). (MGG 100 X) C: Emperiopolesis in megakaryocytes. (MGG 1000 X) D: Hypogranular hypolobated megakaryocyte (MGG 400 X)
All the findings noted in BMA were corroborated with findings in BMB. The number and morphology of the megakaryocytes in non-MDS related thrombocytopenia were assessed. Their significance was studied by comparing with the morphological changes in MDS. The distribution of morphological changes in cases of non-MDS conditions and MDS were compared using Chi-Square test. A p-value less than 0.05 was considered significant. The sensitivity and specificity for those morphological features is not calculated since the data type is not suitable for estimating sensitivities and specificities.
RESULT
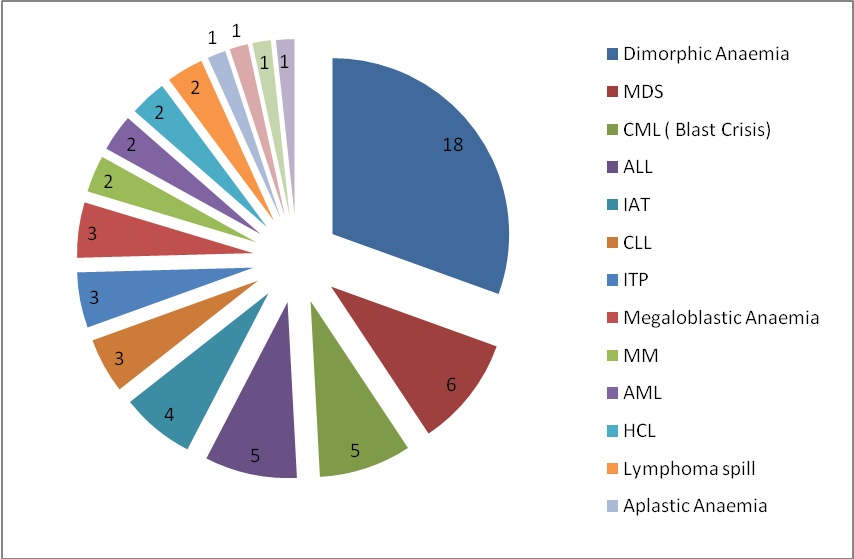
The commonest cause of thrombocytopenia for which bone marrow examination was sought was Dimorphic anaemia (18 cases, 30%). The second most common cause was MDS (06 cases, 10%) which was followed equally by acute lymphocytic leukemia (ALL) and blast crisis of chronic myeloid leukemia (CML). Other causes recorded were multiple myeloma (MM), acute myeloid leukemia (AML), hairy cell leukemia (HCL), infection associated thrombocytopenia (IAT), myelofibrosis (MF), chronic lymphocytic leukemia (CLL), idiopathic thrombocytopenic purpura (ITP), lymphoma spillage, megaloblastic anemia, alastic anemia, hypersplenism and bone marrow necrosis [Table/Fig-3], [Table/Fig-4].
Condition associated with thrombocytopenia in present study
| SN | Conditions | Number | Percentage |
|---|
| 1 | Dimorphic Anaemia | 18 | 30.00 |
| 2 | myelodysplastic syndromes (MDS) | 06 | 10 |
| 3 | Blast crisis Chronic Myeloid Leukemia (CML) | 05 | 8.33 |
| 4 | Acute lymphocytic leukemia (ALL) | 05 | 8.33 |
| 5 | Infection associated thrombocytopenia (IAT) | 04 | 6.66 |
| 6 | lymphocytic leukemia (CLL) | 03 | 5.00 |
| 7 | idiopathic thrombocytopenic purpura (ITP) | 03 | 5.00 |
| 8 | Megaloblastic Anaemia | 03 | 5.00 |
| 9 | multiple myeloma (MM) | 02 | 3.33 |
| 10 | acute myeloid leukemia (AML) | 02 | 3.33 |
| 11 | hairy cell leukemia (HCL) | 02 | 3.33 |
| 12 | Lymphoma spill | 02 | 3.33 |
| 13 | Aplastic Anaemia | 01 | 1.66 |
| 14 | hypersplenism (HS) | 01 | 1.66 |
| 15 | bone marrow necrosis (BMN) | 01 | 1.66 |
| 16 | HD (post chemotherapy) | 01 | 1.66 |
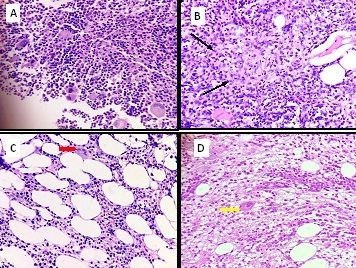
A: Bone marrow biopsy section showing cluserting of megakaryocytes in case of idiopathic thrombocytopenia (ITP). (H& E 400 X) B: Bone marrow biopsy section showing epithelioid cell granuloma in case of a patient with tuberculosis. ( H & E 400 X) C: Bone marrow biopsy section showing increased fat spaces in aplastic anaemia. (H & E 400 X) D: Bone marrow biopsy section showing increased fibrosis in a case of myelofibrosis. ( H & E 400 X)
The changes in number and morphology of megakaryocytes in various hematological disorders were recorded [Table/Fig-5], [Table/Fig-6], [Table/Fig-7] and [Table/Fig-8]. There was an increase in the number of megakaryocytes in 4 cases of dimorphic anaemia and dysplastic forms were seen in 8 cases followed by hypogranular forms and cytoplasmic vacuolization in 6 and 5 cases respectively [Table/Fig-7]. Shows Megakaryocytic alterations observed in 4 commonest hematological disorders causing thrombocytopenia.
Megakaryocytic alterations in different hematological disorders causing thrombocytopenia
| SN | Conditions | Number/Low Power Field |
|---|
| Normal | Increased | Decreased | Absent |
|---|
| 1 | Dimorphic Anaemia | 11 | 04 | 03 | 00 |
| 2 | MDS | 03 | 00 | 03 | 00 |
| 3 | CML (Blast Crisis) | 02 | 01 | 02 | 00 |
| 4 | ALL | 00 | 00 | 05 | 00 |
| 5 | IAT | 02 | 01 | 01 | 00 |
| 6 | CLL | 00 | 00 | 03 | 00 |
| 7 | ITP | 00 | 03 | 00 | 00 |
| 8 | Megaloblastic Anaemia | 01 | 01 | 01 | 00 |
| 9 | MM | 00 | 00 | 02 | 00 |
| 10 | AML | 01 | 00 | 01 | 00 |
| 11 | HCL | 00 | 00 | 02 | 00 |
| 12 | Lymphoma spill | 00 | 00 | 02 | 00 |
| 13 | Aplastic Anaemia | 00 | 00 | 01 | 00 |
| 14 | HS | 00 | 01 | 00 | 00 |
| 15 | BMN | 00 | 00 | 01 | 00 |
| 16 | HD (post chemotherapy) | 00 | 00 | 01 | 00 |
Morphological changes in Megakaryocytes in various conditions
| Conditions | IF | Dysp Forms | Bare forms | EMP | Budding | Cyto Vacuo | MM | Hypo forms | No. of nuclear lobes |
|---|
| N | | |
|---|
| Dimorphic Anaemia | 01 | 08 | 02 | 02 | 00 | 05 | 02 | 06 | 06 | 06 | 13 |
| MDS | 03 | 04 | 01 | 00 | 00 | 00 | 04 | 06 | 04 | 01 | 04 |
| CML ( Blast Crisis) | 01 | 01 | 00 | 00 | 00 | 00 | 04 | 03 | 03 | 01 | 02 |
| ALL | 00 | 00 | 00 | 01 | 00 | 00 | 00 | 05 | 00 | 00 | 05 |
| IAT | 00 | 00 | 00 | 02 | 00 | 02 | 01 | 03 | 01 | 00 | 03 |
| CLL | 00 | 00 | 00 | 00 | 00 | 00 | 00 | 00 | 00 | 00 | 01 |
| ITP | 02 | 02 | 02 | 02 | 01 | 02 | 00 | 01 | 00 | 02 | 03 |
| Megaloblastic Anaemia | 00 | 00 | 01 | 00 | 00 | 00 | 01 | 01 | 02 | 02 | 02 |
| MM | 00 | 00 | 00 | 00 | 00 | 00 | 00 | 01 | 00 | 00 | 02 |
| AML | 00 | 00 | 01 | 00 | 00 | 00 | 00 | 01 | 00 | 00 | 02 |
| HCL | 00 | 00 | 00 | 00 | 00 | 00 | 00 | 00 | 02 | 00 | 02 |
| Lymphoma spill | 00 | 00 | 00 | 00 | 00 | 00 | 00 | 02 | 00 | 00 | 02 |
| Aplastic Anaemia | 00 | 00 | 00 | 00 | 00 | 00 | 00 | 01 | 00 | 00 | 01 |
| HS | 00 | 00 | 00 | 00 | 00 | 00 | 01 | 01 | 01 | 01 | 01 |
| BMN | 00 | 00 | 00 | 00 | 00 | 00 | 00 | 00 | 00 | 00 | 01 |
| HD (post chemotherapy) | 00 | 00 | 00 | 00 | 00 | 00 | 00 | 01 | 00 | 00 | 01 |
Etiology wise distribution of the patients presenting with thrombocytopenia (n=59)
Megakaryocytic alterations observed in 4 commonest hematological disorders causing thrombocytopenia
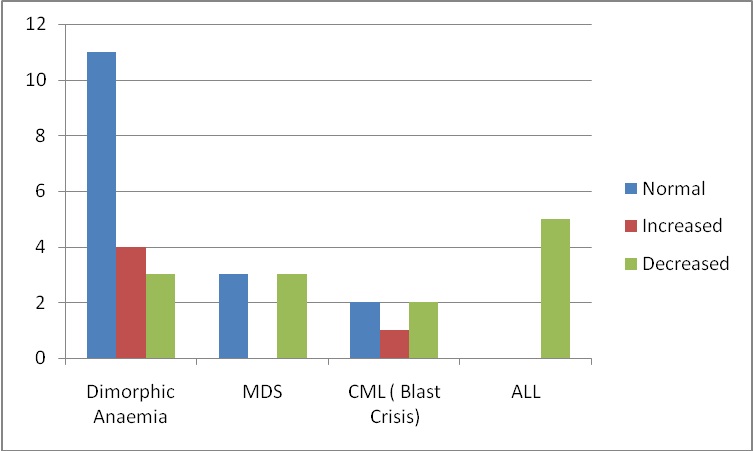
In cases of ALL; apart from hypogranular forms in 5 cases and emperiopolesis(EMP) in 1 case, no major megakaryocytic alteration was detected.
Of all the non-MDS conditions apart from dimorphic anaemia, ITP and CML (blast crisis); megakaryocytic dysplastic froms were not noted in any other condition. On the other hand emperiopolesis was noted in Dimorphic anaemia, IAT and ITP (2 cases each) and one case each in ALL and MF.
In cases of MDS, dysplastic forms, bare megakaryocytic nuclei, hypogranular forms and micromegakaryocytes were seen [Table/Fig-6] [Table/Fig-1 & 2]. The lobes in megakaryocytes were either Normal or reduced in most cases of MDS [Table/Fig-9]. Shows Comparison between frequencies of normal, high and low number of nuclear lobes among MDS (n=9) and non MDS (n=68) conditions.
Comparison between frequencies of normal, high and low number of nuclear lobes among MDS (n=9) & non MDS (n=68) conditions.
| MDS | High | Normal | Low |
|---|
| Chi-Square | 45.208a | 33.779a | 19.753a | 2.195a |
| df | 1 | 1 | 1 | 1 |
| Asymp. Sig. | .000 | .000 | .000 | .138 |
a. 0 cells (.0%) have expected frequencies less than 5. The minimum expected cell frequency is 38.5.
DISCUSSION
MK and platelets, which are their progeny, are highly specialized cells that participate in hemostatic and inflammatory functions. Since each platelet lives only about 10 days, the platelet supply is continually renewed by production of new platelets from the maturation of MK [10].
Megakaryocytic proliferation and differentiation is typically abnormal in patients with myelodysplastic syndromes (MDS) [11]. In the present study a significant difference was noted regarding the MK number among MDS versus non MDS cases (p=0.017) with higher count more indicative of non MDS condition.
Conventionally a normal megakaryocyte has four to sixteen nuclear lobes and an immature megakaryocyte is defined as a young form of megakaryocyte having scant bluish cytoplasm and lacking lobulation of the nucleus which occupies almost all of the cell [6] [Table/Fig-8]. Comparison between frequencies of normal, high and low number of nuclear lobes among MDS (n=9) & non MDS (n=68) conditions can be seen in [Table/Fig-9].
Dysplastic megakaryocytes were defined as those with single/ multiple separate nuclei [Table/Fig-2]. Micromegakaryocytes were defined as megakaryocytes whose size was that of a large lymphocyte/ monocyte and which had a single/bilobed nucleus. The megakaryocytes were considered to show platelet budding if there was budding of cytoplasmic processes from their surfaces. Hypogranular forms were defined as megakaryocytes with pale grey or water clear cytoplasm and sparse or no granules. The type of cell seen within the megakaryocyte in emperipolesis was also documented.
A shift to young, immature, less polypoid megakaryocytes and fewer mature platelet-producing megakaryocytes was the outstanding morphological feature noted in almost all the cases of ITP in the present study. Similar findings were observed by Houwerzijl et al., [9]. Deka L et al., observed that Megakaryocytes in cases of ITP showed a higher nuclear/cytoplasmic ratio (p = 0.021), lower nuclear roundness factor (p = 0.04) and lower nuclear contour ratio (p = 0.027). Cellular circularity and compactness were significantly different in ITP as compared to non-ITP cases, indicating that the megakaryocytes were less round in ITP subjects [12]. Dysplastic forms along with bare forms and micromegakaryocytes were seen in half the cases of ITP.
Emperipolesis, seen in 02 cases with lymphocytes in all cases.
Rai et al reported Emperipolesis in 13 out of 19 cases (68.4%). In 5 cases, lymphocytes were seen within megakaryocytes, 4 cases showed lymphocytes and nucleated red blood cells (nRBCs), 2 cases showed lymphocytes, nutrophils and nRBCs, 2 cases showed nRBCs and one case showed lymphocytes and nutrophils within the megakaryocytes [13]. These findings correlated with the claim of Rozman C. and Vives Corrons JL [14] of a considerable increase in megakaryocytic emperipolesis in idiopathic thrombocytopenic purpura (ITP).
The cytoplasmic vacuolization seen in half of the cases and this reflects an increased megakaryocyte turnover and indicates degenerative changes such as those of apoptosis and para-apoptosis [Table/Fig-3 & 5].
Four case of IAT were observed in our study. Stasi R et al observed that Persistent thrombocytopenia may be the consequence of chronic infections with hepatitis C virus (HCV), human immunodeficiency virus (HIV), and Helicobacter pylori, and should be considered in the differential diagnosis of primary immune thrombocytopenia (ITP) [15]. Three cases of IAT had increased megakaryocytes as noted by Alter, Scanlon and Schechter [16]. According to them, the virus might directly damage the platelets or alter them to become antigenic, resulting in specific antiplatelet antibody formation. Alternatively, a virus-antivirus complex could precipitate on the platelets and damage them resulting in compensatory increase of megakaryocyte in the bone marrow. Other changes which were seen were eperipolesis, cytoplasmic vacuolization, micromegakaryocytes and hypogranular forms [Table/Fig-6].
In Dimorphic anemia, dysplastic forms were seen in eight cases . Wickramasinghe has also observed megakaryocytes with separation of nuclear lobes and nuclear fragments and attributed this to diminished DNA synthesis leading to nuclear maturation defect [17]. The finding of emperipolesis in anemia was in agreement with the observation of Tavassoli [18]. However, no platelet budding was observed in any of the cases.
A single case of hypersplenism showed hypogranular forms along with micro megakaryocytes with no dysplastic changes or nuclear budding seen.these changes are attributed to removal of platelets by increased pooling and by increased phagocytosis in the spleen ( according to Diz-kucukkaya et al., [19].
All the 20 cases of leukemia-lymphoma syndrome showed decreased or absent megakaryocytes. This may be because of the autoantibodies against glycoprotein IIa-IIIb complex which have been demonstrated in patients with lymphoma. According to Lim and Ifthikharuddin, [20] along with immune-mediated platelet destruction, decreased platelet production when the marrow is involved by lymphoma, bone marrow suppression by chemotherapeutic agents and platelet sequestration in the spleen also contribute to thrombocytopenia in lymphoma. Other changes seen are individually tabulated including MF, granulomatous infection and aplastic anaemia [Table/Fig-4].
Further studies on the evaluation of megakaryocytic alteration and their contribution to thrombocytopenia can provide growing knowledge to the pathogenesis of numerous hematopoietic disorders that may identify broader clinical applications of the newer strategies to regulate platelet count and functioning.
a. 0 cells (.0%) have expected frequencies less than 5. The minimum expected cell frequency is 38.5.