A diabetic foot is one of the most feared complications of diabetes and it is the leading cause of the hospitalization among diabetic patients [1]. It is characterized by several pathological complications such as neuropathy, peripheral vascular disease, foot ulceration and infection with or without osteomyelitis, which leads to the development of gangrene and which even necessitates limb amputation. The Indian diabetic population is expected to increase to 57 million by the year 2025 [2]. The individuals with diabetes have at least a 10-fold greater risk of being hospitalized for soft tissue and bone infections of the foot than individuals without diabetes [2].
The impaired micro-vascular circulation in patients with a diabetic foot limits the access of phagocytes, thus favouring the development of an infection [3]. The local injuries and the improper foot wear further compromise the blood supply in the lower extremities [1]. While the foot infections in persons with diabetes are initially treated empirically, a therapy which is directed at the known causative organisms may improve the outcome [4].
Many studies have reported on the bacteriology of Diabetic Foot Infections (DFIs) over the past 25 years, but the results have been varied and often contradictory [4]. These discrepancies could partly have been due to the differences in the causative organisms, which had occurred over time, geographical variations, or the type and the severity of the infection, as were reported in the studies [4]. Mostly, the diabetic foot infections are mixed bacterial infections and the proper management of these infections Sectionrequires an appropriate antibiotic selection, based on the culture and the antimicrobial susceptibility testing results [5].
Now, medical and research communities are beginning to realize that the diversity of the bacterial populations in chronic wounds may be an important contributor to the chronicity of the wounds, such as diabetic foot ulcers. The current study was undertaken as an attempt to examine the major populations of bacteria which were associated with the bio burden of infected diabetic foot ulcers. By performing a survey on the wounds from different subjects, an attempt was made to identify the genera or the noted pathogens that were consistently present in diabetic ulcers [6], and also to note the changes in the bacteriological profiles of the infected foot ulcers, as compared to those which were seen in previous studies. In recent years, there has been an increase in the incidence and the prevalence of ESBLs. Currently, there is a paucity of data on the ESBL-producing and the carbapenemase producing organisms from diabetic foot infections, especially in this part of world [5]. This study was planned with the aim of determining the bacterial profile of infected diabetic foot ulcers and the antibiotic resistance pattern of the bacterial isolates.
MATATERIALS AND METHODS
50 diabetic patients with foot ulcers were included in this study, which was conducted for a period of 6 months. The institutional ethical committee’s clearance was obtained before conducting the study. A clinical history was elicited with regards to the duration of diabetes, the type of treatment which was received and the presence of other systemic illnesses. The patients were also assessed clinically and the ulcers were graded according to Wagner’s grade. The samples were collected after obtaining informed consents from the patients.
Samples were collected from the deeper portion of the ulcers by using 2 sterile swabs which were dipped in sterile glucose broth. The samples were collected by making a firm, rotatory movement with the swabs. One swab was used for Gram staining and the other was used for culture. A direct Gram stained smear of the specimen was examined. The specimens were inoculated onto blood agar, chocolate agar, Mac Conkey’s agar and thioglycollate medium. The inoculated plates were incubated at 370C overnight and the plates were examined for growth, the next day. The further processing was done according to the nature of the isolate, as was determined by Gram staining and the colony morphology. The organisms were identified on the basis of their Gram staining properties and their biochemical reactions.
Antibiotic Susceptibility testing
The antibiotic susceptibility testing was done by the Kirby Bauer disc diffusion method, as per the CLSI guidelines, 2011 [7]. The antimicrobial discs which were used were those of Ampicillin (20μg), Aztreonam (30μg), Gentamicin (10μg), Amikacin (30μg), Cefazolin (30 μg), Cefuroxime (30μg) Ceftazidime (30μg), Cefotaxime (30μg), Ceftriaxone (30μg), Cefepime (30μg), Cefoperazone/sulbactam (75/10μg), Piperacillin/tazobactam(100/10μg), Imipenem (10μg), Meropenem (10 μg), Polymyxin B (300 units) and Colistin (10μg), for the Gram negative bacilli. Penicillin, Ampicillin, Azithromycin (15μg), Cefoxitin (30μg), Cefotaxime (30μg), Chloramphenicol (30μg), Clindamycin (2μg), Erythromycin (15μg), Oxacillin (1μg), Vancomycin (30μg), Teicoplanin (30μg)), Ciprofloxacin, Ofloxacin (5μg), Linezolid (30μg) and Tetracycline (30μg) were used to study the susceptibility patterns of the Gram positive cocci
MRSA, ESBL and carbapenemase production were detected as per the CLSI guidelines 2011 [7].
MRSA detection: The phenotypic test for the detection of MRSA was done by using a cefoxitin (30 μg) disc.
A zone of inhibition which was equal to or more than 22 mm was considered as susceptible to Cefoxitin and the organism was reported as Methicillin Sensitive Staphylococcus aureus. Those isolates which produced a zone of inhibition which was less than or equal to 21 mm were considered as Methicillin Resistant Staphylococcus aureus (MRSA).
ESBL production was confirmed by using discs of Ceftazidime (30 μg) and Ceftazidime Clavulanic acid (30/10 μg) respectively. The test organism was inoculated as a lawn on a Muellar Hinton agar plate and the above mentioned discs were placed on the plate. The plates were incubated at 370C overnight and they were examined next day. An increase in the zone diameter, which was equal to or more than 5 mm for the antimicrobial agent which was tested in combination with clavulanic acid, in comparison to the antimicrobial which was tested alone, indicated that the strain was an ESBL producer.
Carbapenemase production was detected by using the Modified Hodge test. A 0.5 Mac Farland’s suspension of ATCC Escherichia coli 25922, was diluted 1 in 10 in sterile saline. This was inoculated on a Muellar Hinton agar plate, as for the routine disc diffusion testing. The plate was dried for 5 minutes and a disc of Meropenem 10 442μg was placed in the centre of the agar plate. [3–5] colonies of the test organism were picked and inoculated in a straight line, from the edge of the disc, upto a distance of at least 20mm. The plates were incubated at 370C overnight and they were examined next day. They were checked for an enhanced growth around the test organism, at the intersection of the streak and for a zone of inhibition. The presence of an enhanced growth indicated Carbapenemase production, and the absence of an enhanced growth meant that the test isolate did not produce carbapenemase.
RESULTS
In the present study, the age of the patients ranged from 35 to 80 years. The maximum number of patients (20%) was in the age group of 60 to 65 years. The next most prevalent age group was between 50 and 55 years (18%).
A total of 75 bacterial isolates were obtained from 50 patients with diabetic foot ulcers. In this study, gram negative bacilli were isolated more frequently than gram positive cocci. The commonest isolate was Pseudomonas spp (16%), followed by Escherichia coli (14.6%) and Methicillin Sensitive Staphyloccus aureus (13.3%). The other organisms which were isolated were Streptococcus pyogenes (10.6%), Klebsiella spp (8%), Acinetobacter spp (8%), Methicillin Resistant Staphylococcus aureus (MRSA)- 8%, Proteus mirabilis – 6.6%, Citrobacter spp and Enterococcus spp-5.3% each CoNS -2.6% and Enterobacter spp- 1.3%.
In the present study, single organisms were isolated from 25 samples and mixed bacterial growths were seen in 25 samples. The details of the organisms which were isolated from the infected foot lesions have been tabulated in [Table/Fig-1].
Monobacterial & Polybacterial isolates from Diabetic Foot Infections.
| S. No. | Name of the Organism | Total no of isolates -25 | (%) | Name of the Organisms | Total no of isolates -25 | (%) |
|---|
| 1 | Staphylococcus aureus | 4 | 16% | Staphylococcus aureus + Pseudomonas aeruginosa/Klebsiella + MRSA** | 2 each | 8% each |
| 2 | MRSA ** | 2 | 8% | Pseudomonas aeruginosa + MRSA** | 1 | 4% |
| 3 | Streptococcus pyogenes | 4 | 16% | Strepto pyogenes + Staph.aureus | 3 | 12% |
| 4 | Escherichia coli | 4 | 16% | Escherichia coli + Klebsiella/Escherichia coli + Enterococcus/Escherichia coli + Proteus mirabilis /Escherichia coli + MRSA** | 1 each | 4% each |
| 5 | Enterobacter spp | 1 | 4% | Escherichia coli + CoNS | 2 | 8% |
| 6 | Acinetobacter spp | 1 | 4% | Acinetobacter + E.coli/Acinetobacter + Enterococcus spp/Acinetobacter + Citrobacter/Acinetobacter + Pseudomonas aeruginosa/Acinetobacter + Staphylococcus aureus | 1 each | 4% each |
| 7 | Citrobacter | 1 | 4% | Citrobacter + Pseudomonas aeruginosa | 2 | 8% |
| 8 | Pseudomonas aeruginosa | 4 | 16% | Pseudomonas aeruginosa + Enterococcus /Pseudomonas aeruginosa + Proteus mirabilis | 1 each | 4% each |
| 9 | Proteus mirabilis | 2 | 8% | Pseudomonas aeruginosa + Enterococcus/Pseudomonas aeruginosa + Proteus mirabilis | 1 each | 4% each |
| 10 | Enterococcus spp. | 1 | 4% | Pseudomonas aeruginosa + Enterococcus/Pseudomonas aeruginosa + Proteus mirabilis | 1 each | 4% each |
| 11 | Klebsiella spp., | 1 | 4% | Klebsiella sp + Streptococcus pyogenes / Klebsiella spp.,+ Proteus mirabilis + | 1 each | 4% each |
Antibiotic resistance pattern of the isolates
The antibiotic susceptibility patterns of the isolates have been tabulated in [Table/Fig-2] and [Table/Fig-3]. [Table/Fig-2] displays the antibiotic resistance patterns of the gram negative bacilli and [Table/Fig-3] displays the antibiotic resistance patterns of the gram positive cocci. [Table/Fig-4] displays the percentage of the ESBL producers and the carbepenemase producers.
Antibiotic Resistance pattern of Gram Negative Bacilli (% of Resistance).
| Enterobacteriaceae | Non Fermenters |
|---|
| E.coli | Proteus | Klebsiella | Citrobacter | Enterobacter | Acinetobacter | Pseudomonas |
| Amikacin | 0 | 80 | 20 | 50 | 0 | 33 | 15 |
| Ampicillin | 100 | 100 | 100 | 100 | 100 | - | - |
| Aztreonam | 45.4 | 40 | 80 | 60 | 100 | - | 53 |
| Carbenicillin | - | - | - | - | - | - | 8 |
| Cefazolin | 82 | 100 | 100 | 100 | 100 | - | - |
| Cefuroxime | 73 | 80 | 60 | 100 | 0 | - | - |
| Cefotaxime | 73 | 80 | 60 | 100 | 0 | - | - |
| Ceftazidime | - | - | - | - | - | 83 | 61 |
| Cefepime | 45.5 | 40 | 40 | 75 | 0 | 50 | 46 |
| Cefaperazone Sulbactam | 18.1 | 20 | 20 | 25 | 0 | 67 | 0 |
| Ciprofloxacin | 54.5 | 80 | 80 | 75 | 0 | 67 | 46 |
| Chloramphenicol | 0 | 0 | 40 | 100 | 0 | - | - |
| Colistin | 0 | 100 | 0 | 0 | 0 | 0 | 0 |
| Cotrimoxazole | 82 | 100 | 80 | 100 | 0 | 33 | - |
| Gentamicin | 9 | 100 | 100 | 100 | 0 | 50 | 61.5 |
| Imipenem | 0 | 0 | 0 | 25 | 0 | 17 | 0 |
| Meropenem | 45.4 | 40 | 20 | 75 | 0 | 0 | 23 |
| Ofloxacin | 54.5 | 80 | 80 | 75 | 0 | 67 | 53.4 |
| Piperacillin Tazobactam | 27.2 | 0 | 20 | 100 | 0 | 17 | 23 |
| Polymixin B | 0 | 100 | 0 | 0 | 0 | 0 | 0 |
| Tetracycline | 54.5 | 0 | 60 | 0 | 0 | 50 | - |
| Tobramycin | 55 | 40 | 100 | 50 | 0 | 50 | 38.4 |
Antibiotic Resistance pattern of the Gram positive cocci. (% of resistance).
| MSSA | MRSA | CoNS | Streptococci |
|---|
| Penicillin | 100 | 100 | 100 | 37.5 |
| Ampicillin | | | | 50 |
| Cefazolin | 10 | 100 | 100 | 62.5 |
| Cloxacillin | 0 | 100 | | |
| Ciprofloxacin | 50 | 50 | 50 | |
| Ofloxacin | 50 | 50 | 50 | 0 |
| Gentamicin | 10 | 60 | 50 | |
| Netilmicin | 0 | 10 | 0 | |
| Cotrimoxazole | 20 | 40 | | |
| Tetracycline | 50 | 80 | | 0 |
| Erythromycin | 30 | 40 | 50 | 50 |
| Clindamycin | 10 | 20 | 50 | 25 |
| Chloramphenicol | 10 | 20 | 50 | 37.5 |
| Vancomycin | 0 | 0 | 0 | 0 |
| Teicoplanin | 0 | 0 | 0 | 0 |
| Linezolid | 0 | 0 | 0 | 0 |
| Rifampicin | 20 | 40 | 100 | |
Percentage of ESBL producers and Carbapenem Resistant Gram Negative bacilli.
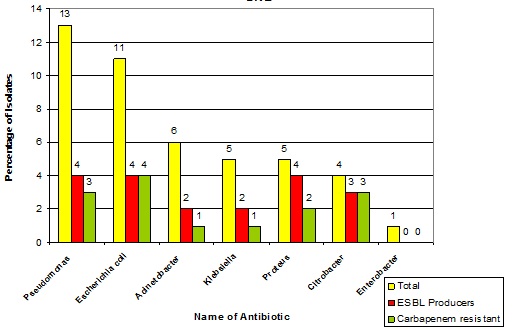
DISCUSSION
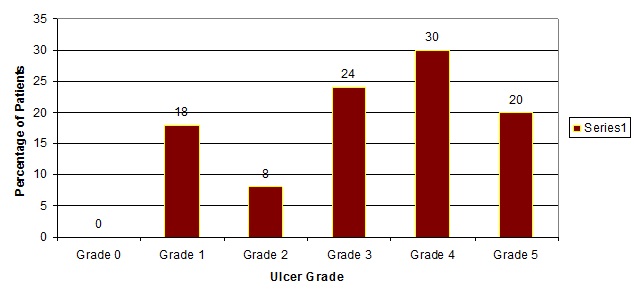
In the present study the maximum number of patients with infected diabetic foot ulcers belonged to Wagner grade 3 and 4 [Table/Fig-5].
Patient distribution according to the grade of the ulcer.
Single bacterial isolate was seen in 50% of the samples and mixed bacterial growth was seen in 50 % of the samples, in our study [Table/Fig-1]. Mohd Zubair et al., [5], Anandi et al., [8], Rama Kant et al., [9], Pappu K et al., [1] and Citron et al., [4] have reported 56.6%, 19%, 23 %, 92% and 16.2 % monomicrobial infections and 33%, 67%, 66%, 7.7% and 83 % of polymicrobial infections respectively. The findings of this study correlate with Zubairs study.
Gram negative bacilli [Table/Fig-1] were more prevalent (65.1%) than gram positive cocci (39.8%). The commonest isolate was Pseudomonas spp (16%), followed by Escherichia coli (14.6%) and Staphylococcus aureus (13.3%). Escherichia coli, Staphylococcus aureus and Pseudomonas aeruginosa were predominant among the monobacterial isolates. Streptococcus pyogenes and Staphylococcus aureus were predominant among the mixed growths. These findings correlated well with those of Pappu K et al., [1], who reported that 76% of the organisms which were isolated were gram negative bacilli, Pseudomonas being the predominant pathogen (23%), followed by Staphylococcus aureus (21%). The reason could be the similar geographical locations where the 2 studies were conducted. Zubair et al., [5] reported Escherichia coli (26.6%) and Pseudomonas aeruginosa (10.6 %) as the predominant gram negative isolates. In the study of Benwan et al., [10] which was done in Kuwait, they reported that more gram-negative pathogens (51.2%) were isolated than gram-positive pathogens (32.3%) or anaerobes (15.3%).
In contrast, Citron et al., [4], Mohd Zubair et al., [5] and Alavi SM et al., [11] reported Staphyloccus aureus as the predominant pathogen, which comprised 57.2%, 28% and 26.2% of their isolates respectively. Streptococcus pyogenes was isolated in 10.6 % of the diabetic foot ulcers. Citron et al., [4], Mohammed Zubair et al., [5] and Ozer. B et al., [12] reported incidences of 15.5%, 6.6% and 6.8% of Streptococcus pyogenes respectively.
Pseudomonas aeruginosa [Table/Fig-3] showed more than 50% resistance to Gentamicin and the Quinolones, 61% resistance to the 3rd generation cephalosporins and 46.1% resistance to the 4th generation cephalosporins. It was sensitive (100%) to Polymyxin B, Colistin, Meropenem and Carbenicillin. Of the 13 Pseudomonas isolates, 4 were Carbapenemase producers as per the Modified Hodge Test [Table/Fig-4].
Tamil Selvi et al., [13], in her study which was done in 2011, reported that the Pseudomonas aeruginosa strains showed 100% resistance to Ampicillin, and Norfloxacin, 83.3% resistance to piperacillin, ticarcillin and tetracycline, 66.6% resistance to ceftazidime, imipenem, gentamicin, amikacin, tobramycin, and cotrimoxazole and 50.0% resistance to cefoperazone. 83.3% of the Pseudomonas aeruginosa strains were susceptible to cefotaxime. These findings did not correlate with our study findings. Shanker et al., has reported that 44% of the Pseudomonas isolates were multi drug resistant [14].
Acinetobacter spp., showed more than 50% resistance [Table/Fig-3] to the Cephalosporins, Quinolones, Penicillins and Tetra-cycline. It was sensitive to Imipenem and Meropenem. Of the 6 isolates, 2 (33.3%) were ESBL producers and 1(16.6%) was a Carbapenemase producer. Acinetobacter spp showed 75.3% resistance to the antibiotics which were tested, in a study which was conducted by Mohammed Zubair et al., [5]. Therefore, the findings of this study did not correlate with those of Zubair’s study.
In our study, the Enterobacteriaceae [Table/Fig-3] showed 100% resistance to Ampicillin, Cefazolin and Gentamicin. All of them were sensitive to Imipenem, except Citrobacter spp. This correlated partly with the findings of a study which was done in Mahatma Gandhi Medical College and Research Institute, Pondicherry, which showed that the members of Enterobacteriaceae were found to be susceptible to Amikacin, Piperacillin Tazobactam and Imipenem [3]. ESBL production was seen in 4 (36.3 %) out of the 11 organisms which were isolated. Carbapenem resistance was seen in 5 (45.4%) isolates. A study which was done in Mustafa Kemal University, Turkey, has documented Enterobacteriaceae as the most frequent bacterial isolates and it reported that most of them were resistant to ampicillin, amoxicillin/ clavulanic acid and cefazolin. Imipenem, meropenem, amikacin and piperacillin/tazobactam were reported as the most effective antimicrobial agents [12]. An earlier study by Prabakar et al., showed that Gram negative aerobic bacilli were sensitive to gentamicin, chloramphenicol, cotrimoxazole and streptomycin [15].
Gadepalli et al., [16] documented that E.coli was the second highest ESBL producer in their study. An increased resistance to cefuroxime and ceftriaxone was noted among the Escherichia coli which were isolated in the study of Sivaraman Umadevi et al., [3].
Among the 5 isolates of Klebsiella pneumoniae, 40% were ESBL producers and 20% were Carbapenamase producers [Table/Fig-4]. Proteus mirabilis [Table/Fig-3] showed 100% resistance to Ampicillin, Cotrimoxazole, Gentamicin and Cefazolin. More than an 80% resistance was noted, to the 3rd and 4th generation cephalosporins, quinolones and amikacin. It was susceptible to chloramphenicol, piperacillin tazobactam, imipenem, meropenem, aztreonam and cefaperazone sulbactam. 80% of the Proteus mirabilis isolates were ESBL producers and 40% were Carbapenemase producers [Table/Fig-4]. Similarly, a study which was done in Mahatma Gandhi Medical College, Pondicherry, reported 62.5% ESBL producing Proteus mirabilis [3].
All the strains of Staphylococci [Table/Fig-5] which were isolated, were resistant to Penicillin and they were susceptible to Vancomycin, Teicoplanin and Linezolid. Syed Mohammed Alavil et al., [11] reported that the Staphylococcus aureus isolates were resistant to all the tested antibiotics, except Ciprofloxacin and Amikacin, of which the sensitivity rates were 91% and 80% respectively. M.B Girish et al., [17] reported that 15% of the MRSA strains were resistant to Ampicillin, Cephalosporins and Gentamicin and that they were sensitive to Amikacin, Vancomycin, Teicoplanin and Linezolid. Raja NS also report that vancomycin was effective against Gram positive cocci [18].
CONCLUSION
Both Gram positive cocci and Gram negative bacilli caused diabetic foot infections and this study showed a preponderance of Gram negative bacilli. There was a variation in the bacterial aetiologies of the DFIs, based on the geographical location. Knowledge on the antibiotic susceptibility pattern of the isolates from diabetic foot infections is crucial for planning the appropriate treatment of these cases, prior to getting the susceptibility reports from the laboratory.