INTRODUCTION
Lithium is a therapeutic agent which is currently in widespread use for the treatment of bipolar disorder [1]. It is a double edged sword; it is a unique drug with an invaluable psychoactive potential on one hand and a drug which can cause multisystem toxicity and even death on the other hand [2]. A frequent side effect of Lithium is renal toxicity. Lithium accumulates in the collecting tubular cells after entering the sodium channels in the transluminal membrane [3]. A major biochemical action of Lithium in the kidney is its competition with magnesium, which thereby inhibits the magnesium dependent G-protein that activates the vasopressin–sensitive, adenylyl cyclase. Lithium induces the down regulation of the vasopressin regulated, water channel, aquaporin-2, which is expressed on the apical plasma membrane of the principal cells of the collecting duct [1]. Nephrogenic Diabetes Insipidus [NDI] is the most common side effect of the Lithium therapy. Lithium toxicity is closely related to the serum Lithium levels and it can occur at doses which are close to the therapeutic levels [4]. As renal excretion is the only route of elimination of Lithium, acute renal failure is a serious complication of an already hazardous condition [5].
The predominant form of chronic renal disease which is associated with the Lithium therapy is a Chronic Tubulointerstitial Nephropathy [CTIN]. The biopsy findings in patients with Lithium induced CTIN include tubular atrophy and interstitial fibrosis, which are typically out of proportion to the degree of glomerulosclerosis or the vascular disease [1].
The mast cells are derived from the haematopoietic progenitor cells. They migrate through the vascularized tissue to complete their maturation [6]. Although the mast cells are found infrequently in the normal kidney tissue, their numbers increase significantly in the setting of renal disease. The mast cells are prominent in tubulointerstitial nephritis which is associated with progressive fibrosis and renal failure. These include almost all the primary and secondary forms of glomerulonephritis, diabetic nephropathy, allograft rejection, amyloidosis, Renovascular ischaemia, reflux nephropathy, polycystic kidney disease and drug induced nephropathy [cyclosporine]. The mast cells can initiate, amplify and direct the innate and the adaptive immune responses. They have diverse functional capacities which are well beyond those which are associated with allergy and immunoglobulin E [IgE] [6]. We are hereby reporting a case of lithium toxicity, with emphasis on the demonstration of the mast cells and its implications.
CASE REPORT
A 41 year old male presented with an altered sensorium of 5 days duration. He was a known case of bipolar affective disorder, who was on Lithium 600mg and resperidone 4mg a day. He was also on anti-hypertensive medication. The duration of the disorder and the treatment could not be elicited. In view of his worsening general condition and his low blood pressure, Lithium and the antihypertensive medications were stopped in the institute in which he was previously admitted. On examination, his pulse rate was found to be 82beats/min and his blood pressure was 150/90mmHg. His respiratory system examination revealed bilateral crepitation on auscultation. Abdomino-pelvic ultrasonography revealed an increased cortical texture of both the kidneys. A computerized tomography scan of the brain revealed diffuse brain atrophy. The haematological investigations revealed a decreased haemoglobin level [11.6g%] and a raised total WBC count [14,500 cells/ mm3]. The differential count revealed neutrophilia. His ESR was raised [110mm/hour]. His urine routine revealed proteinuria (4+), bacteriuria (3+) and numerous pus cells. His urine culture yielded a pure growth of Escherichia Coli with 10,000-20,000 CFUs/ml. The laboratory values of various parameters have been summarized in [Table/Fig-1]. The patient was diagnosed have acute renal failure which was secondary to lithium toxicity. The poor prognosis of the patient was explained to the informant and he was managed with intravenous fluids and antibiotics. The patient’s condition progressively worsened and he expired after 7 days of stay in the hospital. A necropsy was performed.
Biochemical investigations
| Institution | Parameters | Initial value | Last value | Mean |
|---|
| Previous institution | Serum lithium [meq/l] | 2.2 | 0.9 | 1.46 |
| Random blood sugar [mg%] | 104 |
| Creatine phosphokinase | 563 |
| Present institution | Blood urea [mg%] | 103 | 156 | 127 |
| Serum creatinine [mg%] | 3.8 | 6.8 | 5.72 |
| Serum sodium [meq/l] | 169 | 171 | 159.8 |
| Serum potassium [meq/l] | 4.5 | 6.2 | 5.16 |
| Serum chloride [meq/l] | 142 | 147 | 135.6 |
| Blood gas analysis | Compensated metabolic acidosis [Ph 7.32, Po2 28mmhg, HCO3 14.2meq/l] |
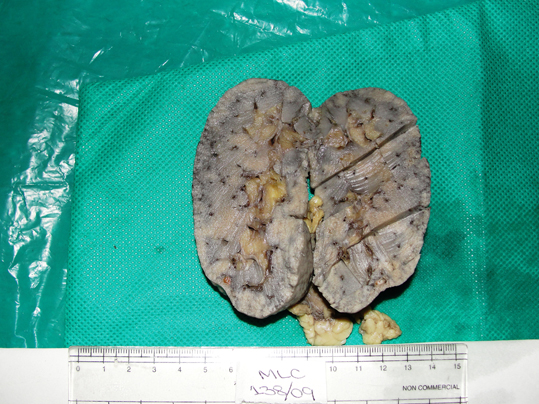
Both his kidneys and two pieces of his lungs were subjected to a histopathological examination. The external surfaces of both the lung pieces showed a anthracotic pigment. The cut section of both the lung pieces showed grey-black to gray-white areas. The right kidney measured 10x4.5x1.5cm. The left kidney measured 10x4.3x1.5cm. The external surfaces of both the kidneys showed a granular appearance. The cut section of both the kidneys showed grey white areas with tiny cysts [Table/Fig-2]. The appearances of the external surface and the cut section were similar to that of the right kidney.
Photograph of cut section of right kidney showing greywhite areas with multiple tiny cysts.
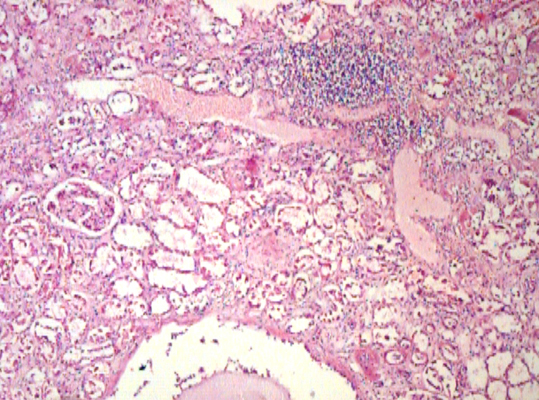
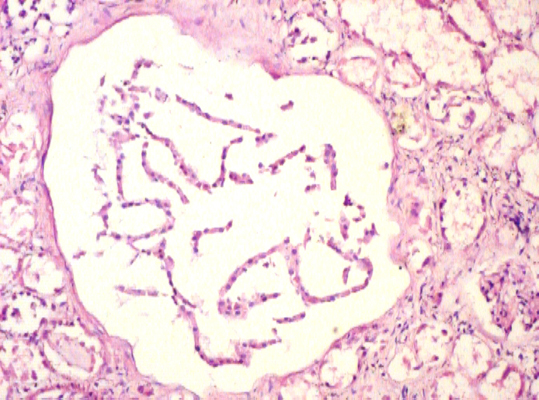
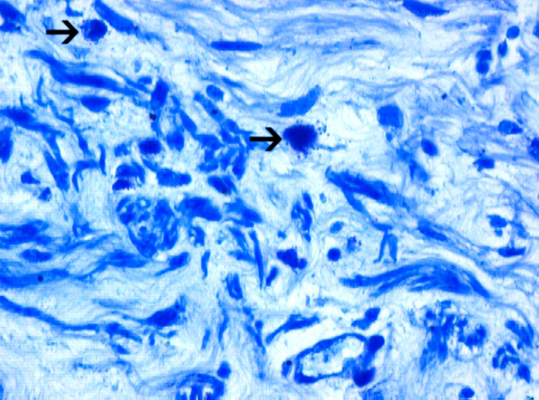
Microscopically, the sections from the lungs showed a congested and an oedematous parenchyma with the features of a patchy pneumonia. The sections from both the kidneys showed focal interstitial mononuclear inflammatory cell infiltration [Table/Fig-3], tubular atrophy and interstitial fibrosis. Tubular cysts [Table/ Fig-4], were seen, a few of which showed desquamation. Some of the tubular cells had large, bizarre, hyperchromatic nuclei. Occasionally, the tubular cells showed hyperchromatic and small pyknotic irregular nuclei and a granular eosinophilic cytoplasm with vacuolation. The glomeruli showed periglomerular fibrosis and focal segmental glomerulosclerosis [FSGS]. Some of the intertubular arteries also showed hyaline arteriolosclerosis. These findings suggested a predominant CTIN with a glomerular and a vascular pathology, which was consistent with the features of lithium induced nephropathy. The Periodic Acid Schiff [PAS] stain demonstrated FSGS. The reticulin stain demonstrated interstitial fibrosis [Table/Fig-5] and periglomerular fibrosis. Toludine blue [1%] staining demonstrated mast cells in the connective tissue of the renal pelvis [Table/Fig-6] and occasionally in the renal interstitium, between the tubules. The mast cells varied in distribution from as high as 14 cells/hpf to one cell/hpf, with a mean of 5.8 cells/hpf. Most of the mast cells were degranulated. In contrast, the sections from the lung tissue showed very few mast cells.
Photomicrograph of renal tissue showing focal interstitial mononuclear cell infiltration [H&E, x40].
Photomicrograph of renal tissue showing tubular cysts [H&E, x200].
Photomicrograph of renal tissue showing interstitial fibrosis [Reticulin, x200].

Photomicrograph of renal tissue showing mast cells [Arrow] in the connective tissue of renal pelvis [Toludine blue, x400].
DISCUSSION
Many clinical and pathological studies have examined the scope of Lithium induced CTIN. The first major analysis that included a pathologic correlation was published in 1977 and it described 14 patients who presented with either an acute intoxication or NDI[1]. All the patients presented with bipolar disorder, with a mean duration of treatment of 13.6yrs [range, 2-25yrs], in a study which was conducted by Markowit GS et al., [1] John Cade recorded the results of the administration of the Lithium salts in ten manic patients; six patients with dementia precox and three melancholics [3]. In the present case, the 41 year old patient was suffering from bipolar disorder, for which he was on Lithium. The durations of the illness and the treatment were not known in the present case. The patient was a known hypertensive in the present case. Markowit GS et al., [1] had observed eight patients with hypertension as an associated condition. In this case, the patient had an altered sensorium with an initial serum lithium level of 2.2meq/L. The neurotoxicity of lithium has been known for a long time. Warick LH et al., [4] reported a case of Lithium poisoning in a patient who presented with an obtunded sensorium and coarse tremors.
The lithium toxicity may occur when the blood levels approach 2meq/L. The lithium toxicity is closely related to the serum lithium levels and it can occur at doses which are close to the therapeutic levels [4]. Lindop GBM et al., [7] observed serum Lithium levels of 1.0 and 1.1meq/L in a patient who presented with Diabetes insipidus, who showed a histological evidence of the unusual damage which was done to the cells which lined the distal nephron.
The external surface of the kidneys had a granulated appearance. Their cut surface revealed multiple, tiny cysts. Hestbech J et al., [8] had made similar observations in their study. Markowitz GS et al., [1] noted CTIN [100% cases], tubular cysts [62.5% cases], tubular dilatation [33.3% cases], FSGS [50% cases] and global sclerosis [100% cases]. The tubulointerstitial disease tended to be patchy, with a geographic zonation. In the present case, all the above mentioned microscopic features were appreciated. FSGS was associated with proteinuria. A similar association was observed in our case. Markowitz GS et al., [1] observed a sparse, predominantly lymphocytic, interstitial infiltrate which was often confined to the areas of interstitial fibrosis. Even in this case, the focal lymphocytic aggregates were observed in the renal interstitium. Hestbech J et al., [8] observed lymphocytes and plasma cells in the fibrous tissue. In their study, one biopsy specimen contrasted sharply with others in being severely infiltrated with neutrophils, lymphocytes, monocytes and plasma cells. The demonstration of mast cells in the present case gave us a clue about the probable role of the mast cells in CTIN, which is the predominant pathological finding which is associated with lithium induced nephropathy.
Roberts ISD et al., [9] demonstrated that the interstitial infiltrate of the mast cells was a consistent feature of renal fibrosis. The number of mast cells which was present, correlated closely with the extent of fibrosis in their study. Most of the mast cells were degranulated in the present case. A similar finding was recorded by Roberts ISD et al., [9] Jones SE et al., [10] in their study on rats, suggested that the profibrotic contents of the mast cells played an active, pathogenetic role in renal fibrosis. In their study, the mast cells were closely associated with the areas of tubulointerstitial fibrosis and tubular atrophy.
The mast cells which were originally named as ‘fattened’ or ‘wellfed’ cells [mast zellen] by Ehrlich, are a sentinel for the host defence [6]. They secrete a range of cytokines which include the interleukins, [IL]-3, IL-4, IL-5, IL-13, IL-6 and IL-8 [9]. The most abundant product of the human mast cells is serine protease tryptase [11]. Kondo S et al., [11] reported for the first time, that tryptase which is secreted by the mast cells may have role in the development of renal interstitial fibrosis, because the number of the infiltrating tryptase positive mast cells was well correlated with the degree of the interstitial scaring. Furthermore, it was observed that the mast cell tryptase was a mitogenic and a fibrogenic factor for the human renal fibroblasts [11]. The mast cells have a role in the immune homeostasis beyond simple allergy. The mast cells have diverse roles which range from pro-inflammatory to immunomodulatory ones [6].
While the mast cells are traditionally known for their roles in the allergic, IgE mediated reactions, there is also a non-immune related mast cell phenotype that predominates in the connective tissue rather than at the mucosal surfaces. These non-immune mast cells participate in the cell differentiation and in particular, in the synthesis of the extra cellular matrix and in the formation of the fibrotic scar tissue [10].
It has been already been demonstrated in an animal model, that cutaneous fibrosis may be inhibited by mast cell stabilizing agents. If an active pathogenetic role for the mast cells in renal fibrosis is demonstrated, these compounds offer the potential for a new approach to the anti-fibrotic treatment, for the management of chronic renal disease [9]. But the initiating factor of the renal fibrosis should be identified. In the present case, the CTIN was due to the lithium therapy. The demonstration of mast cells in the present case, gave us a clue towards their possible role in initiating Lithium induced CTIN. This could be a parallel mechanism in addition to the action of lithium on the collecting tubular cells of the kidney.
CONCLUSION
The present case report reinforces chronic tubulointerstitial nephropathy as a predominant histopathological feature with the constellation of the glomerular pathology and the tubular changes in the lithium toxicity. The demonstration of mast cells in the present case emphasized their possible role as a parallel mechanism in the lithium induced, renal pathology. This case report calls for a study to elucidate and confirm the role of mast cells in the pathogenesis of lithium induced nephropathy. In such a case, an adjuvant treatment with mast cell stabilizers may be beneficial in the patients who receive lithium for retarding the progression of the disease.
[1]. Markowitz GS, Radhakrishnan J, Kambham N, Valeri AM, Hines WH, Agati VD, Lithium Nephrotoxicity: A Progressive Combined Glomerular and Tubulointerstitial NephropathyJ Am Soc Nephrol. 2000 11:1439-48. [Google Scholar]
[2]. Friedman BC, Bekes CE, Scott WE, Bartter T, ARDS Following acute lithium carbonate intoxicationIntensive Care Med. 1992 18(2):123-24. [Google Scholar]
[3]. Johnson G, Lithium-Early Development, Toxicity and Renal functionNeuropsychopharmacology 1998 19(3):200-05. [Google Scholar]
[4]. Warick LH, Lithium Toxicity: Report of a case with Neurologic, Cardiac and Hepatic SequelaeWest J Med 1979 130:259-63. [Google Scholar]
[5]. Lavender S, Brown JN, Berrill WT, Acute renal failure and lithium intoxicationPostgrad Med J. 1973 49:277-79. [Google Scholar]
[6]. Holdsworth SR, Summers SA, Role of Mast Cells in Progressive Renal DiseaseJ Am Soc Nephrol 2008 19:2254-61. [Google Scholar]
[7]. Lindop GBM, Padfield PL, The renal pathology in a case of lithium induced diabetis InsipidusJ Clin Pathol 1975 28:472-75. [Google Scholar]
[8]. Hestbech J, Hansen HE, Amidsen A, Olsen S, Chronic renal lesions following long term treatment with lithumKidney Int. 1977 12:205-13. [Google Scholar]
[9]. Roberts ISD, Brenchley PEC, Mast cells: the forgotten cells of renal fibrosisJ Clin Pathol 2000 53:858-62. [Google Scholar]
[10]. Jones SE, Kelly DJ, Cox AJ, Zhang Y, Gow RM, Gilbert RE, Mast cell infiltration and chemokine expression in progressive renal diseaseKidney Int. 2003 64:906-13. [Google Scholar]
[11]. Kondo S, Kagami S, Kido H, Strutz F, Muller GA, Kuroda Y, Role of Mast Cell Tryptase in Renal Interstitial FibrosisJ Am Soc Nephrol 2001 12:1668-76. [Google Scholar]