Factitious biochemical reports result in the misguiding of clinicians, unnecessary retesting, wrong diagnoses and incorrect treatments. A vigilant biochemist identifies these factitious biochemical reports and alerts the clinician regarding the proper interpretation of the biochemical reports, thus preventing a misdiagnosis and an incorrect treatment. We are presenting a case report of a multiple myeloma patient who presented with factitious biochemical reports which were caused due to paraproteinaemia. In the present case, the patient presented with an underestimation of urea and creatinine, an underestimation of sodium, low albumin levels and high phosphate levels. On repeating the same tests after dilutions and deproteinizing, the effects of the paraproteins on the above mentioned tests were reduced. Thus, from the observations of our study, we suggest that the interference by paraproteinaemia can be reduced by analyzing the biochemical parameters after dilution and deproteinization.
INTRODUCTION
Factitious biochemical reports are one of the common challenges in clinical biochemistry. The errors in the laboratory are preanalytical, analytical and post analytical. A majority of errors lie in the preanalytical phase [1]. The preanalytical phase involves all the various processes before a sample can be measured. The preanalytical factors are parts of the patient-related variables (age, sex, calorie intake, drugs, etc.), the specimen collection (timing of the sample collection, patient identification, the venepuncture techniques, the types of anticoagulants, the specimen volume, etc.), transport of the specimen , the temperature of the sample transport, the specimen processing, the centrifugation technique and the storage conditions.
The analytical factors involve the methods and instruments which are used for measuring the analytes in the specimen, like the factors which are related to the methods which are employed in the analysis, the factors which are related to the calibration of the analyzer which is employed, etc. Finally, the postanalytical factors consist of the reporting and the interpretation of the laboratory results. The laboratory mistakes are principally detected in the preanalytical phase and may lead to further inappropriate investigations or treatments. The icteric, lipaemic or the haemolytic samples which can be detected in the preanalytical phase, are considered as unsuitable for the routine clinical chemistry tests, due to the biological and the analytical interferences. Moreover, the presence of paraproteins interfere in many biochemical measurements. Haemolysis, lipaemia, bilirubinaemia and paraproteinaemia are classified as the most common sources among the endogenous interferents [1].
A significant number of laboratory results and reports are factitious or are misinterpreted by the clinicians and other laboratory clients. Such mishaps may lead to unnecessary diagnostic evaluations and or unwarranted therapeutic interventions [2]. The analytical interference in the clinical laboratory is a well-known phenomenon and it has many different causes. Both exogenous interferents such as drugs and chemical additives or endogenous substances such as haemoglobin, bilirubin and lipids have been well recognized. The interferences which are caused by immunoglobulins are more difficult to test or anticipate. Especially, immunoassays are sensitive to the interferences which are caused by immunoglobulin [3].
CASE REPORT
A 38 years old female came with a history of a decreased urine output of 1 month’s duration, an altered sensorium and fever of 15 days’ duration. She had a history of a hemithyroidectomy which was done 1 year back for a solitary nodule. The patient’s biochemical parameters were as follows : urea - 31 mg/dl, creatinine - 1.2 mg/dl, sodium –143 meq/L, potassium – 4.5 meq/L, chloride – 99 meq/L, albumin – 3.1 g/dl and phosphate – 7.5 mg/dl. After diluting and deproteinizing the serum samples, the blood urea, serum creatinine and the serum sodium levels were found to be elevated, the serum albumin levels were increased and the serum phosphate levels were decreased.
The peripheral smear showed the features of a neutrophilic leukaemoid reaction which was suggestive of sepsis. The patient’s ESR was 148 mm/hour. An immunoglobulin assay which was done, showed that IgG, IgA and IgM were within the normal ranges. Interleukin 6 was increased and procalcitonin was increased, which were suggestive of sepsis.
A light chain assay showed the lamda chain to be higher than the kappa chain. Parathormone (PTH) was 14.7pg/ml(normal range 10-55pg/ml) and Vitamin D was 4.3ng/ml (normal range 20-40 ng/ml).Serum protein electrophoresis showed the following –Albumin – 47%, Alpha 1 – 5.9 %, Alpha 2 – 17.6 %, Beta – 5.9 % and Gamma – 23.8 %.
The gamma region of the serum protein electrophoresis showed the M band [Table/Fig-1].
Gamma region of serum protein electrophoresis showed M band

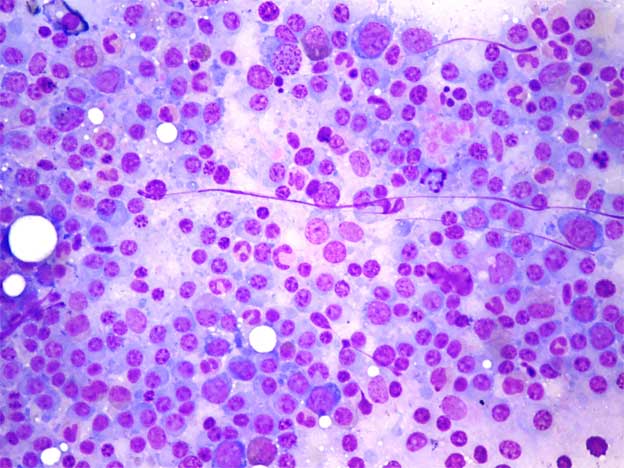
A bone marrow trephine biopsy showed sheets of atypical myeloma cells with prominent nucleoli (plasmablasts) – Haematoxylin and Eosin, 400 X [Table/Fig-2].
Bone marrow trephine biopsy showed sheets of atypical myeloma cells with prominent nucleoli (plasmablasts) – Haematoxylene & eosin, 400 X
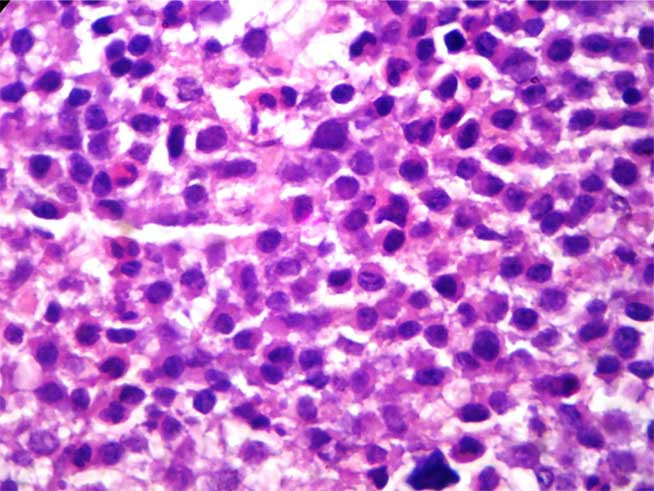
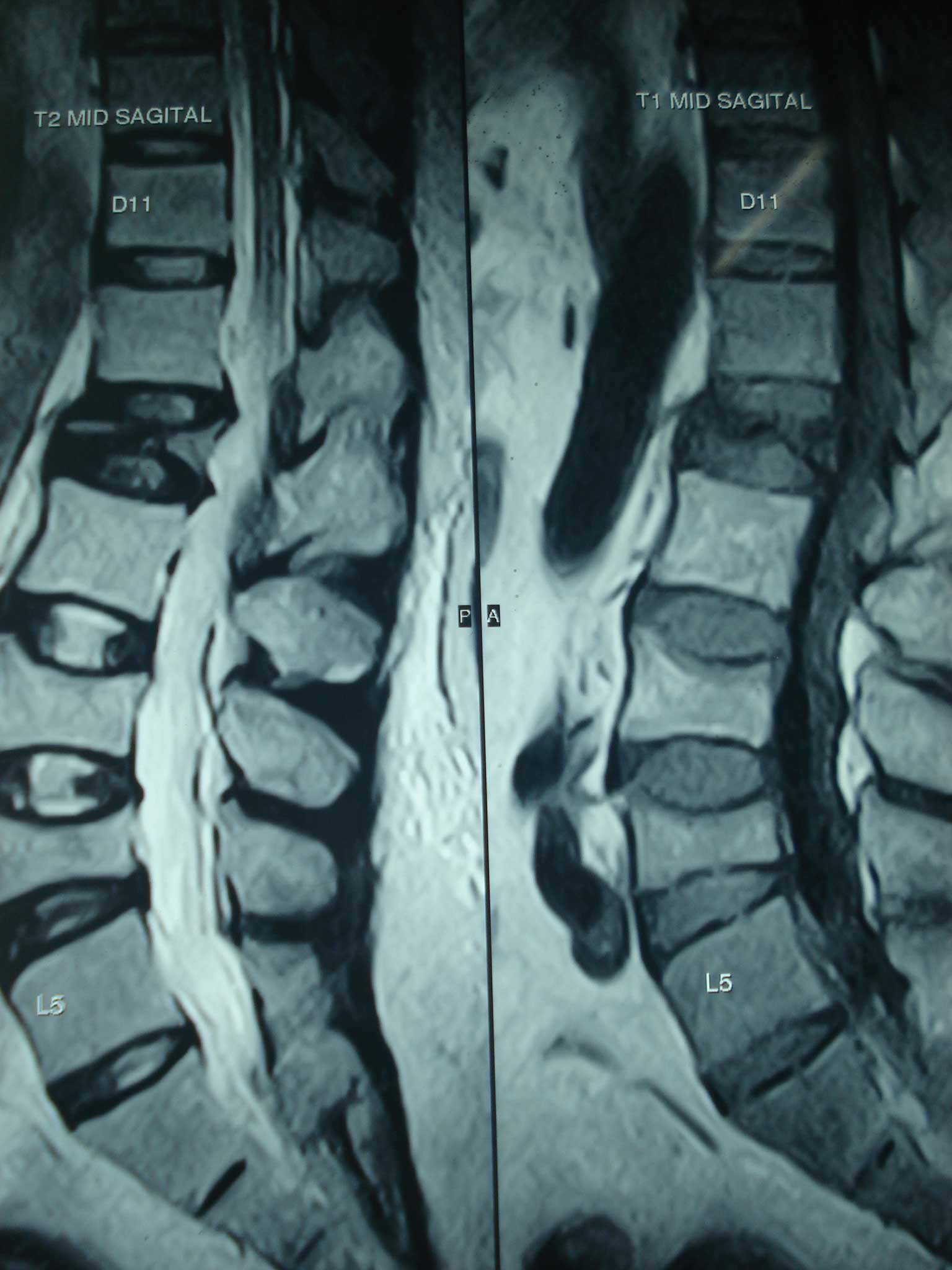
The bone marrow aspirate showed sheets with numerous atypical myeloma cells (plasmablasts). The bone marrow aspiration showed 70 % myeloma cells – Wright-Giemsa, 100 X [Table/Fig-3]. Ultrasound of the abdominopelvis showed bilateral medical renal disease. A chest X-ray showed bilateral reticulonodular opacities in both the lung fields. CT scan of the chest showed a consolidation in the bilateral lung fields and the nodal opacities in the lung were prominent, which were suggestive of myeloma of the lung, no mediastinaladenopathy, multiple lytic lesions in the left ribs and the spine (T9-T12) and suspicious lytic lesions in the right side scapula. T2W (A) and T2W(B) mid sagittal Magnetic Resonance Imaging (MRI) of the spine showed a wedge compression fracture with an altered signal at the L1-L3 vertebral bodies [Table/Fig-4]. CT scan of the abdomen showed hepatosplenomegaly with suspicious hypoechoic lesions in the left lobe of the liver. Ascitis was present, with no retroperitoneal adepathy. A small X-ray (lateral view) of the skull which was done, was within normal limits. The patient did not respond to IV antibiotics and IV dexamethasone.
Bone marrow aspiration showed 70 % myeloma cells – Wright-Giemsa, 100 X
T2W (A) and T2 W(B) mid sagittal magnetic resonance imaging (MRI) of spine showed wedge compression fracture with altered signal at L1-L3 vertebral bodies
DISCUSSION
Monoclonal immunoglobulins, also known as M proteins or paraproteins, can be found in about 1% of the subjects who are over 50 years and the prevalence increases with advancing age to more than 3% in persons who are over 70 years. They are associated with a wide range of clinical manifestations which include B-cell lymphoproliferative disorders such as multiple myeloma (Kahler’s disease) and lymphoplasmacytic lymphoma [3–6]. Studies have shown that paraproteins interfere with the estimation of parameters like CRP, Ferritin, HDL-Cholesterol, glucose, bilirubin, highdensity lipoprotein (HDL), sodium, chloride, phosphate, calcium, urate, thyroxine, antistreptolysin-O (ASO), urea, creatinine, and albumin [2,7]. In our study, we observed that paraproteins caused an underestimation of urea and creatinine, an underestimation of sodium, low albumin levels and high phosphate levels, which were determined after analyzing the same parameters after diluting and deproteinizing the serum samples.
The immunoglobulins which are associated with the factitious results are usually monoclonal, but they can also be polyclonal [2]. Among the paraproteins, IgM is more often the culprit, because its higher molecular weight yields heavier precipitates and causes more interferences. In some cases, the investigation of the clinically “out of place” factitious biochemical results leads to the identification of a paraprotein and the subsequent diagnosis of an underlying lymphoplasmacytic neoplasm [8,9]. The frequency of the immunoglobulin-induced laboratory errors is variable and probably underreported. Large reviews which were done on the patients with paraproteinaemia showed that the immunoglobulin concentration in the patients with the factitious results did not differ from those in the patients without the interference. When the interference occurred, however, the degree of interference was proportional to the immunoglobulin levels [2].
Immunoglobulins can induce factitious results in one or more of the following ways: (1) They may precipitate or flocculate due to a chemical interaction with the test reagents or with their inherent chemical structure, eg, cryoglobulinaemia. The opaque precipitates interfere with automated nephelometric, turbidimetric, colorimetric, and light-scatter–based autoanalyzers. (2) Immunoglobulins may chemically inactivate the test reagents. (3) Immunoglobulins increase the colloid phase of plasma and relatively decrease the aqueous phase, thereby reducing the amount of the water-soluble molecules. and (4) Immunoglobulins may act as antigens and bind to the reagents or to the analyte and interfere with the immunologic measurements. The paraprotein interference in the laboratory testing is intermittent; it is seen in some patients and not in others and at one time in a patient and not at another time. Also, since the reagents are frequently modified by the manufacturers, these laboratory errors are difficult to anticipate [2].
The paraprotein-associated factitious results can be minimized or avoided by taking one or more of the following measures: (1) diluting the sample to reduce the precipitates; (2) “deproteination” of the sample to eliminate the paraprotein; (3) using a saline or a water blank instead of plasma (IgM, but not IgG or IgA, interferes with the reading of the blank cuvettes in some instruments) and (4) setting up manual flags that identify the higher baseline absorbance or a sudden change in the absorbance when the interacting reagent is added [2,10,11]; such manual flags are, however, discouraged by the instrument manufacturers.
Thus, we conclude that factitious biochemical findings do occur in the patients with paraproteinaemias and that they can be reduced by dilution and deproteinization.
[1]. Piyophirapong Sudarat, Wongtiraporn Wanida, Sribhen Kosit, Factitious Results in Clinical Chemistry Tests Caused by Common Endogenous InterferentsSri Raj Med Journal. 2010 62:185-88. [Google Scholar]
[2]. Bakul I, Dalal MD, Brigden Malcolm L, Factitious Biochemical Measurements Resulting From Hematologic ConditionsAm J Clin Pathol. 2009 131:195-204. [Google Scholar]
[3]. Berth M., Delanghe J, Protein precipitation as a possible important pitfall in the clinical chemistry analysis of blood samples containing monoclonalimmunoglobulins:2 case reports and a review of the literatureActa Clinica Belgica 2004 :59-65. [Google Scholar]
[4]. Grogan TM, Van Camp B, Kyle RA, Müller-Hermelinck HK, Harris NL, Plasma cell neoplasms. In: Jaffe ES, HarrisNL, Stein H, Vardiman JW, edsTumours of haemopoietic and lymphoid tissues, WHO classification of tumours 2001 LyonIARC Press:142-56. [Google Scholar]
[5]. Criteria for the classification of monoclonal gammopathies, multiple myeloma and related disorders: a report of the International Myeloma Working GroupBr J Haematol 2003 121:749-57. [Google Scholar]
[6]. Owen RG, Treon SP, Al-Katib A, Clinicopathological definition of Waldenstrom’s macroglobulinemia: consensus panel recommendations from the Second International Workshop on Waldenstrom’s macroglobulinemiaSemin Oncol. 2003 30:110-15. [Google Scholar]
[7]. Lau LG, Chng WJ, Liu TC, Transfusion medicine illustrated: unnecessary transfusions due to pseudothrombocytopeniaTransfusion 2004 44:801 [Google Scholar]
[8]. Wenk RE, Yoho S, Bengzan A, Pseudohypoglycemia with monoclonal immunoglobulin M [letter]Arch Pathol Lab Med. 2005 129:454-55. [Google Scholar]
[9]. Kadri N, Douville P, Lachance P, Monoclonal paraprotein may interfere with the Roche direct HDL-C Plus assay [letter]Clin Chem. 2002 48:964 [Google Scholar]
[10]. Pantanowitz L, Horowitz GL, Palakalin JN, Artifactual hyperbilirubinemia due to paraprotein interferenceArch Pathol Lab Med 2003 127:55-59. [Google Scholar]
[11]. Smogorzewska A, Flood JG, Long WH, Paraprotein interference in automated chemistry analyzersClin Chem. 2004 50:1691-93. [Google Scholar]