Lichenoid eruptions represent a heterogeneous group of conditions that resemble idiopathic Lichen Planus (LP) in terms of their clinical appearance and demonstrate a lichenoid tissue reaction. The latter (as defined by Pinkus) is a histologic pattern which is characterized by an epidermal basal cell damage that is intimately associated with a massive infiltration of mononuclear cells in the papillary dermis. Many clinically distinct inflammatory dermatoses have in common, varying elements of lichenoid histologic features, and these are referred to as lichenoid dermatoses [1].
The spectrum of clinical diseases which is related to the lichenoid tissue reaction is wider. Most of the components of the lichenoid spectrum exhibit basal cell damage and a band-like lymphocytic infiltrate that hugs the dermo-epidermal junction, except for subtle differences that define the particular variant. The prototype of all the lichenoid eruptions is Lichen planus [2].
Certain disorders are common in children, whereas others more often affect the adult population. Lichen striatus, Lichen nitidus, the Gianotti-Crosti syndrome, and Lichen spinulosus are examples of lichenoid lesions that are more common in children than in adults [3].
It is necessary to distinguish these diseases for the prediction of the course of the eruption and for an optimal management. In most of the cases, certain clinical characteristics enable the clinician in reaching a diagnosis, whereas in other cases, a biopsy may be required to get a definitive answer. Many of these lesions are self-limited and they only require symptomatic treatment, although corticosteroids can hasten the resolution in certain disorders. Discontinuation of the medication is often sufficient for the resolution of the lichenoid drug eruptions [4].
A combination of the histologic details, in correlation with the clinical data, help in arriving at a more specific diagnosis.
MATERIALS AND METHODS
This was a one and a half year prospective study which was conducted in the Dermatopathology Section of the Department of Pathology from January 2011 to June 2012.
All the patients who visited the Dermatology Outpatients/Inpatients Department and presented with lichenoid tissue reactions were included in the study. The selected patients’ clinical findings were noted and their informed consents were taken after explaining to them the details of the biopsy procedure. The biopsies were taken from the lesions along with the surrounding areas. The biopsy areas were cleaned and painted with an antiseptic solution and an adequate amount of local anaesthetic (2% lidocaine) was injected into the skin and the subcutaneous tissue. The specimens were preserved in 10% formalin. A gross examination was done and the sections were embedded in paraffin. A light microscopy technique was used for the diagnosis.
All the skin biopsies with a clinical diagnosis of lichenoid tissue reactions at our hospital, were included in the study which was done from Jan 2011 to June 2012. The SPSS, version 14 software was used to analyze the data. Frequencies and percentages were used to describe the data.
Each skin biopsy was subjected to systematic and critical interpretive assessments in the sequence of the epidermal changes, like basal cell death or vacuolar change and the varying thickness of the different layers of epidermis.
The dermal changes like interface dermatitis and the compositions of the different cell types, the focal or diffuse nature of the lesions and the pigment incontinence, along with the appendageal involvement, were noted.
RESULTS
During this study period, 107 cases were clinically diagnosed as lichenoid reactions, of which 84 cases were concordant on histopathology, 23 were discordant and 6 cases were diagnosed, solely based on the histology [Table/Fig-1]. So, a total of 90 cases were diagnosed histologically, of which 42 were of the Lichen planus type and 48 were lichenoid eruptions.
Clinicopathological Correlation
| Correlation | Number of cases | Percentage |
|---|
| Clinico-Pathologically concordant | 84 | 78.50% |
| Clinico-Pathologically discordant | 23 | 21.50% |
| Cases diagnosed only on histology | 06 | -- |
Of the 90 patients, 38 were males and 52 were females. The youngest was a 1 year female and the oldest was a 71 year male [Table/Fig-2]. Females were affected more commonly than the males. Spectrum of histopathological changes in epidermis and dermis were also studied [Table/Fig-3,4]. On histopathological examination, epidermal changes [Table/Fig-5] like acanthosis and liquefactive degeneration of the basal layer and dermal changes [Table/Fig-6] like a dense, band like lymphocytic infiltration and melanin incontinence were seen in most of the patients. Civatte bodies were seen in only 19 patients (21.1%).
Showing Age and Sex distribution
| Age (Yrs) | Male | Female | Total | Percentage (%) |
|---|
| 1-10 | 11 | 14 | 25 | 27.7% |
| 11-20 | 18 | 18 | 36 | 40% |
| 21-30 | 02 | 07 | 10 | 11.11% |
| 31-40 | 03 | 01 | 04 | 4.44% |
| 41-50 | 01 | 06 | 07 | 7.77% |
| 51-60 | 01 | 03 | 04 | 4.44% |
| 61-70 | 01 | 03 | 04 | 4.44% |
| 71-80 | 01 | 00 | 01 | 1.11% |
| Total | 38 | 52 | 90 | 100 |
Showing histopathological diagnosis of various lichenoid reactions of skin
| Diagnosis | No. of patients | Percentage |
|---|
| Lichen Planus |
| Classical Lichen planus [Table/Fig-7] | 24 | 26.66% |
| Lichen planus pigmentosus | 06 | 6.66% |
| Follicular lichen planus [Table/Fig-14,15] | 05 | 5.55% |
| Actinic lichen planus [Table/Fig-8] | 03 | 3.33% |
| Hypertrophic lichen planus | 02 | 2.22% |
| Eruptive lichen planus | 02 | 2.22% |
| lichenoid eruptions |
| Lichen sclerosis | 04 | 4.44% |
| Lichen sclerosis et atrophicus | 07 | 7.77% |
| Lichen spinulosus | 02 | 2.22% |
| Lupus erythematosus [Table/Fig-12,13] | 09 | 10% |
| Lichen nitidus [Table/Fig-10,11] | 05 | 5.55% |
| Lichen striatus [Table/Fig-9] | 04 | 4.44% |
| Drug induced | 03 | 3.33% |
| Pityriasis lichenoids (Chronic & Acute) | 05 | 5.55% |
| Erythema Multiformi | 05 | 5.55% |
| Poikiloderma | 03 | 3.33% |
| Lichen planus like-keratosis | 01 | 1.11% |
| Total | 90 | 100 |
Showing spectrum of histopathological changes in epidermis and dermis in various lichenoid reactions of skin
| Sl.No | Epidermis | Cases (90) | Sl.No | Dermis | Cases (90) |
|---|
| 1 | Parakeratosis | 06 | 1. | Band like configuration hugging the basal layer | 84 |
| 2 | Hyperkeratosis | 84 | 2. | Mostly lymphocytes | 90 |
| 3 | Acanthosis | 75 | 3. | Melanin incontinence | 84 |
| 4 | Atrophy | 14 | 4. | Plasma cells | 08 |
| 5 | Spongiosis | 61 | 5. | Eosinophils | 04 |
| 6 | Papillomatosis | 22 | | | |
| 7 | Saw toothed rete ridges | 54 | | | |
| 8 | Civatte bodies | 19 | | | |
| 9 | Liquefactive degeneration of basal layer | 87 | | | |
| 10 | Max-Joseph spaces | 09 | | | |
| 11 | Follicular plugging | 12 | | | |
Comparison of epidermal changes with other study
| Sl.No | Features | Present study | Ellis Francis (1965)[8] |
|---|
| 1 | Parakeratosis | 6.6% | 12% |
| 2 | Acanthosis | 83.3% | 23% |
| 3 | Atrophy | 15.5% | 47% |
| 4 | Civatte bodies | 211% | 37% |
| 5 | Liquefactive degeneration of basal layer | 96.6% | 100% |
| 6 | Max-Joseph space | 10% | 17% |
Comparison of dermal changes with other study
| Sl.No | Features | Present study | Ellis Francis (1965) [8] |
|---|
| 1 | Band like configuration hugging basal layer | 93.3% | 100% |
| 2 | Mostly lymphocytes | 100% | 100% |
| 3 | Plasma cells | 8.8% | 3.0% |
| 4 | Eosinophils | 4.4% | -- |
| 5 | Melanin Incontinence | 93.3% | -- |
So, a total of 90 cases were diagnosed histologically, of which 42 were of the Lichen planus type and 48 were lichenoid eruptions [Table/Fig-7, 8, 9,10,11,12,13,14 and 15].
Photomicrograph showing wedge shaped hypergranulosis, basal cell vacuolation, melanin incontinence and band like lymphocytic infiltration (Classical Lichen Planus). [H & E 20X]
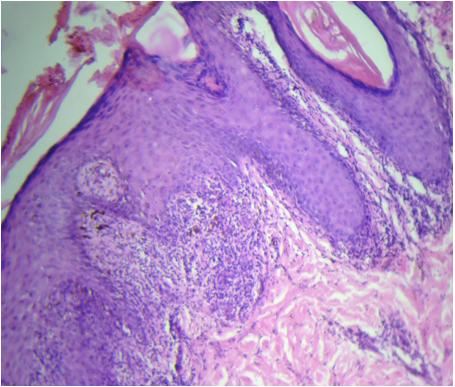
Photomicrograph showing thinned out epidermis at the centre with basal cell vacuolation, melanin incontinence and lymphocytic infiltration (Actinic Lichen Planus). [H & E 20X]
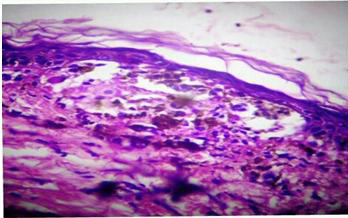
Photomicrograph showing parakeratosis, basal cell vacuolation and dense lympho-histiocytic infiltration with exocytosis of lymphocytes (Lichen striatus). [H & E 20X]
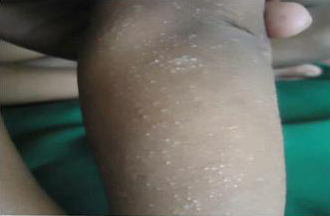
Photograph showing numerous tiny papules over lower extremities (Lichen Nitidus)
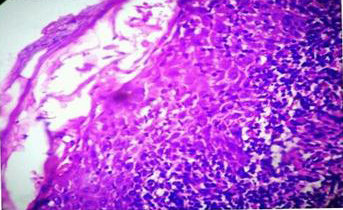
Photomicrograph showing basal cell vacuolation with rete ridges clutching the lymphocytic infiltrate in the manner of a “claw clutching a ball”. (Lichen Nitidus) [H & E 10X]
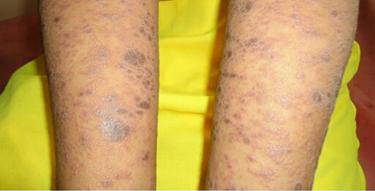
Photograph showing raised, violaceous plaques(Lupus erythematosus)
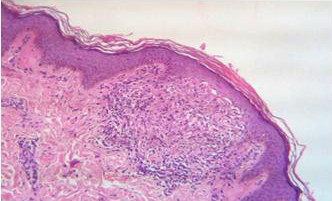
Photomicrograph showing thinned out epidermis with dense dermal lymphocytic infiltration and melanin incontinence (Lupus erythematosus)[H & E 10X].
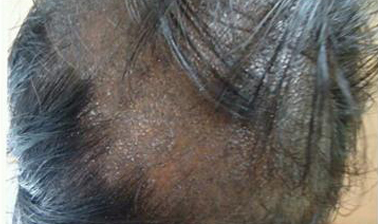
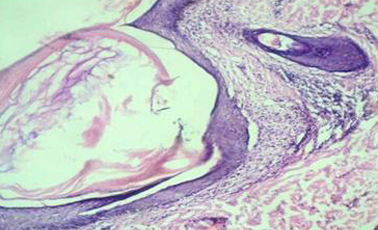
Photograph showing flat, toppled violaceous papules with fine scales over scalp (Lichen Planopilaris)
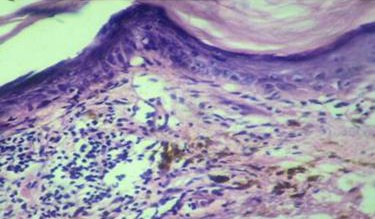
Photomicrograph showing follicular plugging with dense peri-infundibular lymphocytic infiltration (Lichen Planopilaris)[H & E 10X].
The lichenoid reactions were more common in children and the most common prototype was Lichen planus. The most common locations which were involved were the upper extremities. The cases which were diagnosed only on the basis of the histology were of the Lichen planus type.
Among the 90 clinical cases which were concordant on histopathology, 42 were of the Lichen planus type and 48 were lichenoid eruptions.
Among this Lichen planus type, there were 24 cases of classical Lichen planus and 18 were other morphological (histopathological) variants of Lichen planus. Among the lichenoid eruptions, there were 4 cases of Lichen sclerosis, 7 cases of Lichen sclerosis et atrophicus, 2 cases of Lichen spinulosis, 9 cases of lupus erythematosus, 5 cases of Lichen nitidus, 4 cases of Lichen striatus, 3 drug induced, 4 PL chronic, 5 erythema multiformi, 3 poikiloderma and one case each of PLEVA and Lichen planus like keratoses.
DISCUSSION
The term “lichenoid” refers to the papular lesions of certain skin disorders, of which lichen planus (LP) is the prototype. The papules are shiny, flat-topped, polygonal and of different sizes, and they occur in clusters, creating a pattern that resembles that of the lichen which grows on a rock. On histologic examination, the lichenoid lesions are characterized by an infiltrate of inflammatory cells that fills the papillary dermis in a band-like fashion and often obscures the dermo-epidermal junction. In addition, there is a vacuolar degeneration of the basal layer of the epidermal keratinocytes [3,4].
The histologic findings that typify the lichenoid disorders are thought to reflect the underlying mechanism in which there is an immune mediated attack on the basal keratinocytes, which might explain both the presence of an inflammatory cell infiltrate and the vacuolar degeneration of the basal keratinocyte layer [5].
Most of the cases in our study were seen in the younger age group (1-30yrs), which was comparable with the findings of Sehgal et al., [6] (11-40yrs). The youngest was a 1 year female and the oldest was a 71 year old male. In our study, of the 90 patients 38 were males and 52 were females. There was a male/female ratio of 0.73 which was comparable with the findings of Singh and Kanwar [7] 0.50.
It is essential to distinguish Lichen planus from other lichenoid dermatosis on microscopy, because they have got different modalities of treatment and prognosis. A lichenoid exanthem (e.g., triggered by a drug-induced reaction) can resemble exanthematous Lichen planus. Psoriasis punctata can also raise a suspicion of Lichen planus. Lichen ruber anularis may mimic morphea or erythemas annulare, and Lichen ruber linearis may resemble a striated nevus (inflammatory linear verrucous epidermal nevus; ILVEN) or Lichen striatus. These may be ruled out on the basis of the patient’s medical history and the typical predilection sites. In Lichen ruber of the palms of the hands and the plantar surfaces, the clinical appearance resembles rhagadiform and/or hyperkeratotic hand/foot eczema [9].
There is a therapeutic importance in differentiating hypertrophic LP from Lichen planus, because rarely does squamous cell carcinoma develop in the lesions of long standing hypertrophic LP and it has also been reported in several patients who are infected with the human immunodeficiency virus and the HCV infection. Castleman’s tumour (giant lymph node hyperplasia) and malignant lymphoma are rare associations of erosive Lichen planus. Oral squamous cell carcinoma is a rare complication of oral Lichen planus. Lichen planus pigmentosus has been reported in association with a head and neck cancer and with concurrent acrokeratosis paraneoplastica [10].
The prognosis is generally favourable, although the patient’s quality of life is severely impaired by the often intense pruritus. Exanthematous Lichen planus resolves with therapy. The tendency to relapse is low, at 20%. The local forms, however, tend to persist for a very long time. These include oral Lichen planus and Lichen ruber verrucosus (up to seven years) [9].
A general rule on the duration of the disease: generalized Lichen planus (LP) shows a longer duration of the disease as compared to Lichen planopilaris. Generalized LP < cutaneous LP< mucocutaneous LP < mucous LP< Hypertrophic LP< Lichen planopilaris [9].
CONCLUSION
Distinguishing various lichenoid eruptions can be a challenge for a dermatologist. The differential diagnosis can often be narrowed by focusing on the distinctive histopathological features of the various lichenoid eruptions. So, we conclude that histopathology is a dependable tool for identifying the underlying cause in lichenoid eruptions, for an early diagnosis and the appropriate treatment.