The ingestion of contaminated, undercooked food or water is the main cause of acquiring campylobacteriosis. The most common symptoms which are associated with the C.jejuni infection are diarrhoea of abrupt onset, which is accompanied by severe abdominal pain, with the passage of >8 stools on a single day and fever of >40oC. Faecal leukocytes and RBCs are detected in the stools of 75% of the infected persons [6]. Among the most serious complications, the Gullain-Barré Syndrome, the Miller Fisher Syndrome (acute polyneuropathy) and the Reiter’s Syndrome (reactive arthropathy) are remarkable. There is only little recent data of the association of the Campylobacter spp. with paediatric diarrhoeal patients and the organism’s antibiotic resistance pattern in the Indian population. An alarming recent trend is the rapid global emergence of Campylobacter strains which are resistant to the fluoroquinolones (FQ) and the macrolides, which are the most frequently used antimicrobials for the treatment of campylobacteriosis. The ciprofloxacin resistance in the C. jejuni isolates in Thailand was reported to be as high as 100%, while less than 5% of the C. jejuni and 14–36% of the C. coli isolates were resistant to the macrolides [7]. Their changing antimicrobial resistance patterns are an important public health issue.
MATERIALS AND METHODS
Stool samples were collected from 350 children (who were aged ≤12 years) who presented with acute diarrhoea (< 14days duration), who were admitted to the paediatric diarrhoea ward of Lok Nayak Hospital, New Delhi, India, from September 2010 to April 2012. After giving proper counselling, an informed consent was taken from the parents/guardians/persons who attended to the study subjects. A detailed personal history, the diarrhoeal episodes and the associated signs and symptoms were recorded on a predesigned proforma. The children who were on antimicrobial therapy were excluded from the study.
The samples were directly inoculated onto modified Charcoal Cefoperazoned esoxycholate agar (CCDA) (Oxoid®, Hampshire, United Kingdom) with a Campylobacter selective supplement (Butzler) (Oxoid®, Hampshire, United Kingdom), which contained bacitracin (12,500IU), cycloheximide (25mg), colistinsulfate (5,000IU), cephazolin sodium (7.5mg) and novobiocin (2.5mg) and a growth supplement (Oxoid®, Hampshire, United Kingdom). The incubation was done for 48 hours at 42°C under microaerophilic conditions (5% O2, 5% CO2, 2% H2, and 88% N2 by volume) by using the ANOXOMAT AN2OP system (Mart Microbiology®, Drachten, Netherlands). The suspected, moist, translucent colonies were identified by using a modified Gram’s stain and the oxidase test.The isolates quinowere speciated by using biochemical tests such as the catalase test, hippurate hydrolysis, growth on 1% glycine and 1.5% NaCl and susceptibility to nalidixic acid (30ug). The antimicrobial susceptibility testing of all the isolates was carried out by using the Kirby- Bauer disk diffusion method. Bacterial suspensions were made in Mueller-Hinton broth upto a density equivalent of a 0.5 MacFarland’s turbidity standard. Mueller Hinton agar which was enriched with 5% sheep blood (in house) was used for performing the test. Nine antimicrobial agents, namely, amoxicillin (25μg), cefotaxime (30μg), gentamicin (10μg), amikacin (30μg),tetracycline (30μg), chloramphenicol (30μg), erythromycin(15μg), ciprofloxacin(5μg), and nalidixic acid(30μg)(HiMedia®, Mumbai) were tested according to the CLSI guidelines [8].
RESULTS
A total of 36 (10.28%) children with diarrhoea were positive for the Campylobacter infection by the culture method. The mean age of the children with the Campylobacter infection was 9 months, with a peak incidence (18.96%) in the children who were below 1 year of age. All the 36 isolates of C. jejuni were tested for their antimicrobial susceptibilities by using the disc diffusion technique [Table/Fig-1].
Antimicrobial Resistance Profile of Campylobacter Isolates
NA, nalidixic acid; Cipro, ciprofloxaxin; Tet, tetracycline; Ery, erythromycin; Cefo, cefotaxime; Genta, gentamicin; Amika, amikacin; Chlor, Chloramphenicol; Amox, amoxicillin.
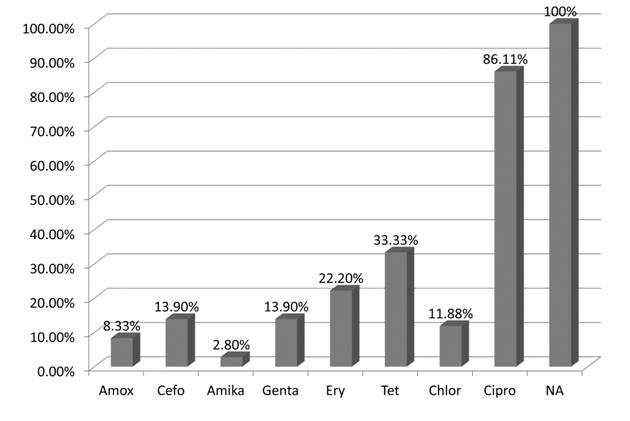
All the isolates were resistant to nalidixic acid and most (86.11%) were resistant to ciprofloxacin. The resistance to tetracycline was 33.33%, that to erythromycin was 22.2%, that to both gentamicin and cefotaxime was 13.9%, that to chloramphenicol was 11.88% and that to amoxicillin was 8.33%. Only one isolate was found to be resistant to amikacin.
Multi-drug resistance (resistance to three or more agents) was found in 15/36 (41.66%) isolates [Table/Fig-2]. The most common combinations which showed drug resistance were nalidixic acidciprofloxacin- erythromycin-tetracycline (n=4) and nalidixic acidciprofloxacin- amoxicillin-cefotaxime (n=3).
Resistance Patternsof Campylobacter Isolates(N = 36)
| Profile | No. of isolates* |
| No resistance demonstrated | | 0 |
| Resistance to one agent (n=13, 36.11%) | NA/Cipro | 11 |
| NA alone | 2 |
| Resistance to two agents (n=8, 22.22%) | NA/Cipro-Tet | 2 |
| NA/Cipro-Ery | 1 |
| NA/Cipro-Cefo | 1 |
| NA/Cipro-Genta | 1 |
| NA/Cipro-Amika | 1 |
| NA/Cipro-Chlor | 1 |
| NA-Genta | 1 |
| Resistance to three agents (n=13, 36.11%) | NA/Cipro-Ery-Tet | 4 |
| NA/Cipro-Amox-Cefo | 3 |
| NA/Cipro-Ery-Chlor | 2 |
| NA/Cipro-Tet-Amox | 1 |
| NA/Cipro-Tet-Genta | 1 |
| NA/Cipro-Genta-Cefo | 1 |
| NA/Cipro-Tet-Cefo | 1 |
| Resistance to four agents (n=2, 5.55%) | NA/Cipro-Tet-Genta-Ery | 1 |
| NA-Genta-Tet-Chlor | 1 |
DISCUSSION
Campylobacter species are primarily zoonotic, with a wide variety of wild and domestic animals, especially birds implicated as reservoir [1]. Campylobacter gastroenteritis is especially common among children who are less than 5 years of age, with an isolation rate of around 57.6% in the developing countries [9]. Studies done in Kolkata and Bangladesh showed that children below 12 months of age were predominantly infected [10,11]. Living with animals under the same roof, the presence of uncovered garbage in cooking areas, lack of a safe water supply and lack of knowledge about the sanitary disposal of faeces are the main risk factors behind the acquiring of this infection. The consumption and handling of chicken as a primary source of the Campylobacter infection have been documented in a previous Indian study [12]. A high prevalence (48% to 64%) of C. jejuni in the chicken samples, which was detected in different parts of India, incriminated poultry as the reservoir of human campylobactereriosis [4,12]. Most typically, the infection with C. results in an acute, self-limited, gastrointestinal illness which is characterized by diarrhoea, fever and abdominal cramps. Antimicrobial use in both humans and animals may be the most important factor for the development of bacteria, with increased resistance and virulence.The rate of resistance to these drugs in the developing countries, which is higher as compared to that in the developed countries, may be due to their use for infections other than gastroenteritis and self medication.
A previous Indian study reported a high resistance (71.4%) of the Campylobacter spp. to ciprofloxacin and only 6.1% resistance to erythromycin [5]. In our present study, a similar percentage (86.11%) of ciprofloxacin resistance was detected but a greater (22.2%) resistance to erythromycin was identified. The use of fluoroquinolones in the veterinary practice as growth promoters indiscriminates the use of this antimicrobial and this may explain the dramatic rise in resistance among the Campylobacter strains. Till date, erythromycin is the drug of choice for campylobacter gastroenteritis, and the low resistance which was shown in our study emphasises its future usefulness for the treatment of this infection. Multidrug resistance is not rare in Campylobacter. Jain et al., found multidrug resistance in 30.6% of the strains [5]. A high prevalence of the multi drug resistant strains of C. jejuni which originated from humans, broilers and pigs were also reported from southeast Asia. Most often, the strains were resistant to nalidixic acid, ciprofloxacin and tetracycline.
It was also observed in many studies, that the duration of diarrhoea, the hospital stay and the risk of invasive illness or death were influenced by the antimicrobial resistance of the Campylobacter isolates [13,14]. Hence, a high resistance to the quinowere lones and the macrolides and multidrug resistance warrant the reconsideration of their use as the drugs of choice in patients with severe gastroenteritis, when Campylobacter is the presumed cause [15].
Overall, the present study provides the recent trend of the antimicrobial susceptibility of the C. jejuni isolates in children, which would be helpful for clinicians to start an empirical therapy for the acute diarrhoea cases. The demonstration of a rising resistance pattern for most of the antimicrobials, reinforces the fact that the indiscriminate use of antimicrobials, both in the human and the veterinary fields, should be controlled. Antibiotic policies need to be strictly adhered to, to prevent further worsening of the situation. The implementation of a surveillance on the susceptibility pattern of the Campylobacter isolates from chicken, which can transfer resistant strains to humans, is highly recommended.