The Human Immunodeficiency Virus (HIV), the Hepatitis B Virus (HBV) and the Hepatitis C Virus (HCV) have common modes of transmission, namely sexual, parenteral and prenatal, the most common being sexual transmission. A history of multiple sex partners, irregular condom use by the clients, and co-infection with other STIs constitute the potential risk factors which are associated with the HIV infection among the FSWs [1]. The Hepatitis B and C viral infections are highly prevalent among the HIV-infected persons, generally as a result of the shared transmission routes, namely sexual, parenteral and perinatal, the most common one being sexual transmission [2]. The HIV screening is done routinely; however, the screening for hepatitis viruses remains somewhat neglected.
Hence, we carried out this study to determine the prevalence of these infections in the FSW population, as well as to find the coinfection rates among this population.
MATERIALS AND METHODS
This study was conducted after obtaining permission from the institutional ethics committee. The samples were collected during October to December 2007. The tests were performed during 2008. The data composition and analysis were done in 2009-10.
The sample size: A 250 sample size was determined by the formula, n=Z2 p (1-p)/d2, where n= sample size, z= 1.96 for95% confidence limit, p= prevalence rate and d=degree of precision [3].
The serum samples were collected from 250 Female Sex Workers from an STI clinic in a red light area by using an outreach strategy, over a period of three months i.e. October- December 2007. They were tested on an unlinked anonymous basis after obtaining an informed consent from the subjects.
Inclusion criteria: According to the UNAIDS definition, the women who receive money or goods in exchange for sexual services, either regularly or occasionally and those who may or may not consciously define these activities as income generating, are female sex workers [4]. The subjects who visited the clinic for the first time during the study period, were included in the study.
Exclusion criteria: The repeat visitors were excluded.
The following details were noted in the proforma: Age, literacy status and the presence of any STD symptoms.
The samples were tested for the HIV antibodies by using Microlisa (J. Mitra and Co, New Delhi) and Retrocheck (Qualpro Diagnostics, Goa, India) as per the Strategy II of the NACO guidelines [4], for the anti HCV antibodies by using the Innova HCV ELISA Kit (Span Diagnostics, Surat, India), for HBsAg by using the Microscreen HBsAg ELISA kit (Span diagnostics, Surat, India) and for the Reagin antibodies by RPR (Carbogen, Tulip diagnostics, Goa ). The positive samples were confirmed by retesting by the respective ELISA tests.
Ethical consideration: This study was conducted after obtaining the approval of the institutional ethics committee.
Statistical analysis: It was done by using the Chi square test.
RESULTS
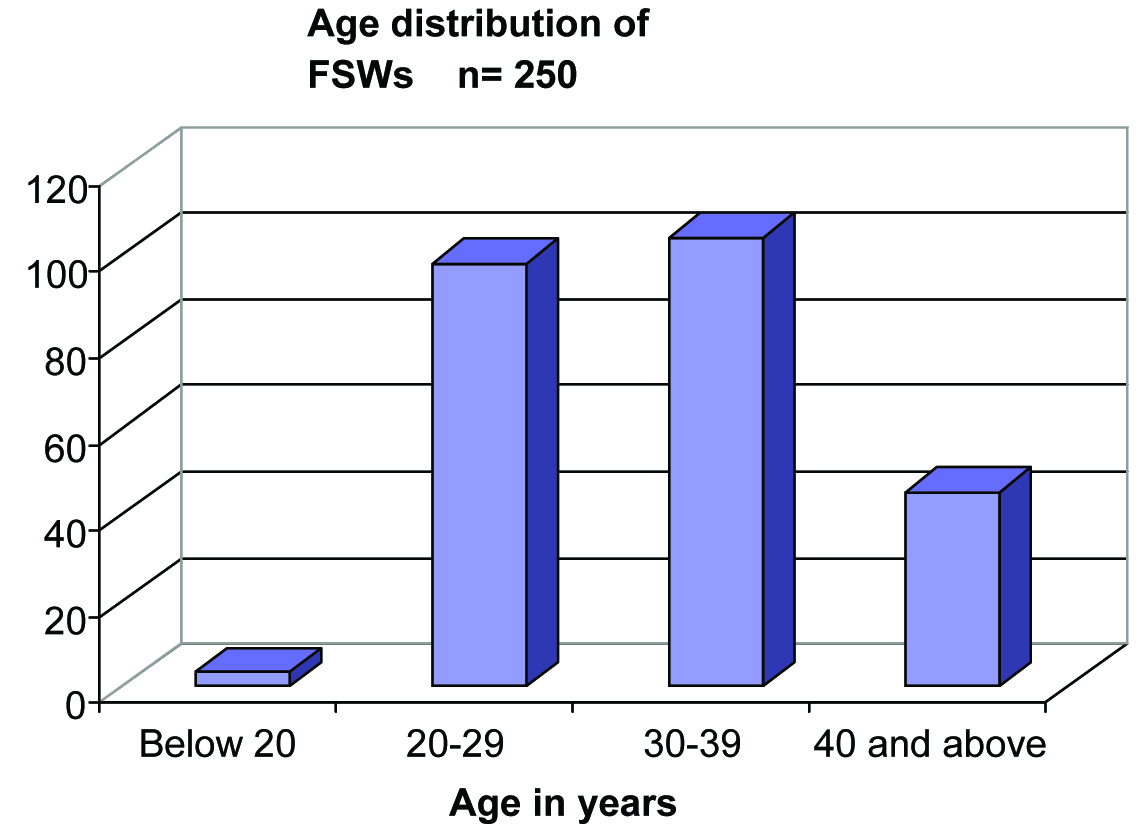
The ages of the 250 FSWs in our study ranged between 17 to 48 years. Three were less than 20 years and 204 were between 20-39 years of age. The mean age of FSWs was 31 years with an S. D. of 6.52 [Table/Fig-1].
Age distribution among the FSWs (n=250)
234 (93.6%) of the FSWs did not have the symptoms of STDs. [Table/Fig-2].
symptoms of STD among FSWs
| STD Symptoms | Positive | Percentage |
|---|
| Asymptomatic | 234 | 93.6% |
| Genital ulcer | 02 | 0.8% |
| Urethral discharge | 13 | 5.2% |
| Urethral ulcer & urethral discharge | 2 | 0.8% |
| Genital warts | 0 | 0 |
| Total | 250 | |
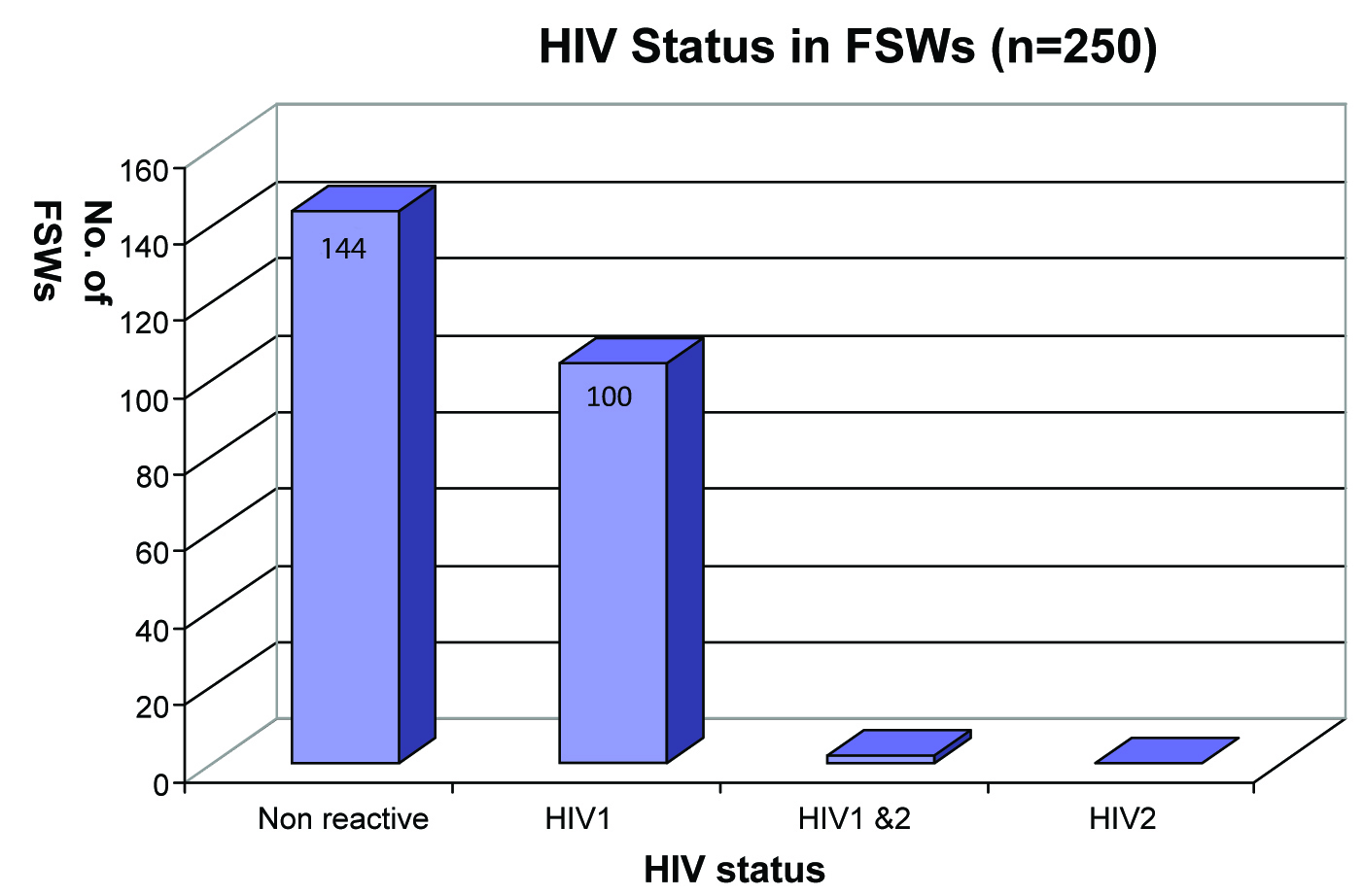
106 (42.4%) of the FSWs were HIV reactive; Most of them were infected with HIV1 [Table/Fig-3].
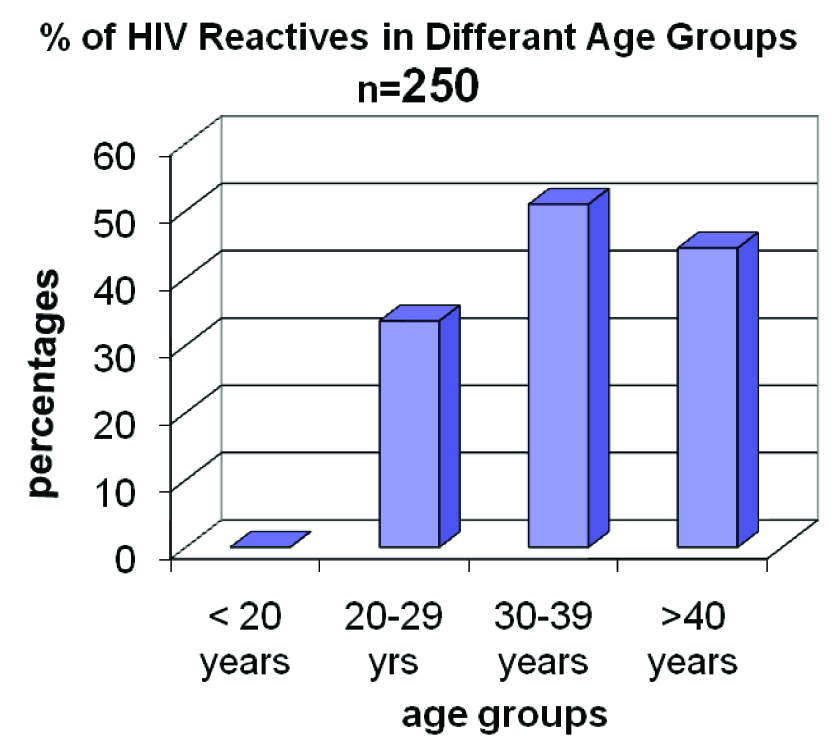
In 30-39 age-group, 50% FSWs were HIV reactive [Table/Fig-4].
HIV reactivity among different age groups
9 out of 17 (53%) of the symptomatic STD patients were HIV positive. All the patients with urethral ulcers and urethral discharges were HIV reactive [Table/Fig-5].
STD symptoms in HIV reactive FSW’s
| STD Symptoms | Total | HIV Reactive |
|---|
| Genital ulcer | 02 | 01(50%) |
| Urethral discharge | 13 | 06(46.15%) |
| Urethral ulcer & urethral discharge | 02 | 02(100%) |
| Total | 17 | 09(53%) |
X2(2, n=17) = 2.025, p=.3633 No statistical correlation was found between the STD symptoms and the HIV reactivity.
15 (6%) of the FSWs were RPR Reactive. Only one FSW with a symptomatic STD was RPR reactive. She presented with a urethral discharge.
19 (7.6%) of the FSWs were HBsAg reactive. A majority of the HBsAg reactive FSWs were in the age group of 20-29 years. All the HBsAg reactive FSWs were non-migrants and none of them had studied beyond standard V. Only 1 of the FSW’s who had symptoms of an STD , was HBsAg positive. She had presented with urethral ulcers and discharge.
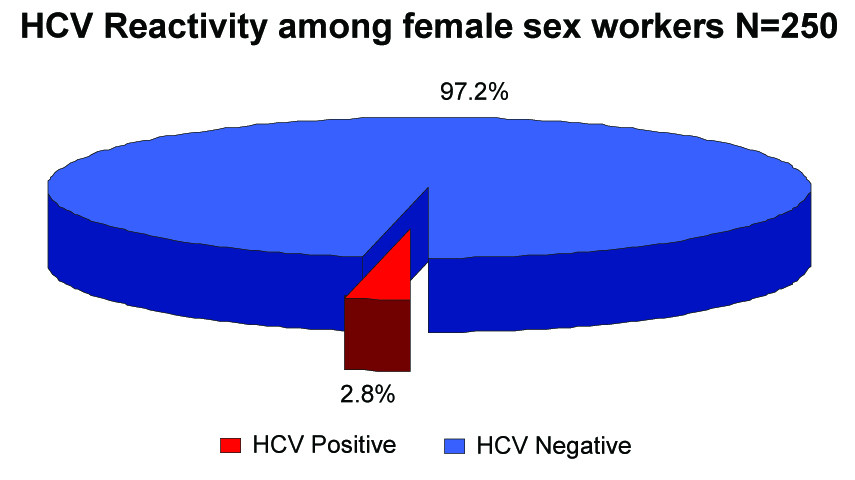
Seven (2.8%) FSWs were HCV reactive [Table/Fig-6].
HIV reactivity among different age groups
Most i. e. 12 out of the 15 (80%) RPR reactive FSWs were also HIV reactive. A majority were in the 20-40 year age group and they were illiterate. Only 11% of the HIV positive patients were RPR reactive. HIV and RPR reactivity was seen in 12 out of the 250 (4.8%) FSWs. corrected Chi square statistics=6.649. degree of freedom: 1 and p=0.009. It was statistically significant [Table/Fig-7].
HIV, HBSAG, HCV & RPR reactivity (N=250)
| HIV (+) | HIV(-) | Total |
|---|
| RPR (+) | 12 (4.8%) | 03 (1.2%) | 15 (6%) |
| RPR (-) | 94 (37.6%) | 141 (56.4%) | 235 (94%) |
| HBsAg (+) | 07 (2.8%) | 12 (4.8%) | 19 (7.6%) |
| HBsAg (-) | 99 (39.6%) | 132 (52.8%) | 231 (92.4%) |
| Anti-HCV (+) | 3(1.2%) | 4 (1.6%) | 7 (2.8%) |
| Anti-HCV (-) | 103 (41.2%) | 140 (56%) | 243 (97.2%) |
| Total | 106 (42.4%) | 144 (57.6%) | 250 (100%) |
An HIV and HBV co-infection was found in 7 (2.8%) out of the 250 female sex workers [Table/Fig-8].
HBV & RPR reactivity in female sex workers (N = 250)
| HBsAg (+) | HBsAg (-) | Total |
|---|
| RPR (+) | 2(0.8%) | 104(41.6%) | 106 |
| RPR (-) | 17(6.8%) | 127(40.8%) | 144 |
| Total | 19 | 231 | 250 |
Corrected Chi square statistics=0.072.degree of freedom: 1 and p=0.7883. This test was not statistically significant.
The HIV and HCV co-infection rate was 1.2% in the female sex workers. Chi square value: 0.132, degree of freedom: 1 and p=0.7166. This test was not statistically significant.
Two out of the 250 i.e. 0.8% FSWs showed HBV and RPR Reactivity.
Corrected Chi square statistics:7.1 degree of freedom: 1 and p=0.0073. This test was not statistically significant.
None of the FSWs were co-infected with HBV and HCV. None had all the three infections.
DISCUSSION
A history of multiple sex partners, irregular condom use by the clients, and co-infection with other STIs constitute the potential risk factors which are associated with the HIV infection among the FSWs [1]. The Hepatitis B and C viral infections are highly prevalent among the HIV-infected persons, generally as a result of the shared transmission routes, namely sexual, parenteral and perinatal, the most common one being sexual transmission [2].
HIV modifies the history of HBV and HCV. To complete the list, HBV and HCV remain a major cause of the hospital admissions and death in the HIV infected patients [5].
Due to multiple sex partners, the FSWs normally have high rates of different sexually transmitted infections, which include HIV. Also, their clients tend to have other high risk behaviours like illicit drug use, etc, thus increasing the risk of acquiring blood-borne viruses. In addition to this, the FSWs themselves may be intravenous (IV) drug abusers [1]. The reduction of the HIV incidence in the FSWs leads to a reduction of the same in the general population [6].
Though HBV, HCV and HIV share common routes of transfer, they differ in their prevalence by the geographical area and the entities by which certain types of exposures transmit them [7].
Different studies have reported different rates of infections among the FSWs. Unsafe vaginal sex increases the risk of acquiring HBV. While becoming infected sexually for HCV is less likely than that for HBV[8–11].
Our study on the high risk group of FSWs was aimed at identifying the factors that could influence the transmission of these 4 STIs. Similar studies were conducted at various places in India and abroad. The mean age of the FSWs in our study was 31 years, which was comparable with that in other studies which were conducted in India and other developing countries. One of the most important factors which forced women into commercial sex work was illiteracy and our study showed that 88% of our subjects were illiterate. Most of the FSWs denied a history of any STD symptoms, which may be due to taboo.
The 42% HIV reactivity among the FSWs was very high. Such high rates were also found in the Indian state of Karnataka and in rural Cambodia. An Italian study showed a lower rate of 4.6% [12]. This difference may be due to the different population dynamics. Most of the HIV reactive FSWs were in the 30-39 years age group. Studies which were done in Cambodia and Nigeria also showed the major affected age group to be the 25-35 years age group [13,14].
The 6% RPR reactivity among the FSWs was similar to that which was found in a study in Surat [15]. But higher rates i. e. 31.8% in Kakinada, 22% in Peddapurum [16] were reported. A lower rate i.e. 3.73% was found in BJMC, Ahmedabad [17]. Such a varied RPR reactivity was also seen worldwide. In Zanzibar, during 2006-7, the prevalence of Syphilis was 1.3% [18].
It varied from 14.2% in the US- Mexico border cities to 45.7% in Argentina[1,12,18–22]. This varied prevalence rates may be due to the different population characteristics.
HIV and RPR reactivities were seen in 4.8% of the subjects. It was statistically significant. It suggested that Syphilis significantly increases the risk of acquiring HIV. The HIV and RPR reactivity in various Indian studies ranged from 0.5% in Dibrugarh to 14.28% in Gujrat, India [15].
The 8% HBsAg reactivity was comparable to the 9.9% in the Kakinada study [16]. Among the other Indian studies, it ranged from 3.33% in Surat[15] to 12.2% in Peddapuram[16]. Similarly, it ranged from 3.5% in Italy[12], to 5.3% in Zanzibar[17], to 14.3% in Argentina [1], to 62% in Hongkong [22]. This may be due to the differences in the geographic locations and the population behaviours, as well as the risk factors like IV drug abuse.
The HIV-HBsAg reactivity was 2.8% in our study. It was lesser as compared to those in other studies i.e. 5.71% in a Surat study [15] and 12.2% in an Argentina study [1].
In our study, 0.8% of the FSWs showed HBV and RPR reactivities. Most of the studies did not find any coinfection. But it was higher ie. 7.5% in Argentina [1]. However, this study was conducted in FSWs by using IV drugs.
The HCV reactivity was 2.8% among the female sex workers in our study. Similar rates were found in Zanzibar (2%) [18] and Peru (2%) [23]. A lesser rate was found in Italy (0.9%) [12]. Higher rates were found in Argentina (4.3%), where IVDU was common among the FSWs [1]. The HIV and HCV co-infection rates were 1.2% each in the female sex workers. Similar low rates (0.5%) were found in Peru [23]. A very high coinfection rate of 30% was found among the Argentinian FSWs who had a very high rate of IV drug abuse [1]. Thus, the varied co-infection rates may be due to the differences in the major risk factors which were associated with the HCV infection i. e. IV drug abuse, illegal blood transfusions, etc. Hence, our study reconfirmed that the HIV infection itself does not increase the risk of the HCV infection.
None were found to be HBV and HCV co-infected or infected with all HIV, HCV or HBV.
The HIV and the HBV infections coexisted among the female sex workers. The seroprevalence of HCV was not significantly high, thus suggesting that the heterosexual route was not a major mode of transmission for HCV. Thus, the varied co-infection rates may be due to the differences in the major risk factors which were associated with the HCV infection i. e. IV drug abuse, illegal blood transfusions, etc. HIV was significantly associated with the RPR reactivity. The HIV-HBV and the HIV-HCV coinfection rates were low in our study. No significant association was found between the HIV, HBV and the HCV co-infections. But the high prevalence rates could be due to the high risk behaviour of this population group. Hence, there is a need to screen the FSWs for the HBV and the HCV infections.
CONCLUSION
There is a need to strengthen the HIV prevention programmes in the FSW population with an emphasis on the IEC activities, condom promotion, the early diagnosis and treatment of the STIs; needle exchange programmes, etc. HBV has a very effective vaccine. Hence, all FSWs, high risk groups and HIV positive individuals should be offered the HBV vaccination. The Hepatitis viral markers should be tested in the HIV positive individuals. In the absence of an effective treatment for these 3 diseases, prevention remains the key solution for the patient protection. Hence, these high risk groups need to have access to preventive measures, not only for HIV but also for HBV and HCV.