Topical corticosteroids are the most frequently used drugs in the dermatology practice. Topical applications help in delivering the drug directly to the target organ and thus the dose can be titrated according to the response, which can be easily monitored. For maximizing the efficacy and the safety of the topical corticosteroids, various factors have to be taken into account, which include the nature and the extent of the skin disease, the age of the patient, the site which is affected, the pharmacokinetic properties of the corticosteroids, the vehicle which is used, the application frequency and the duration and the use of the occlusion. The concentration, the dose of the drug and the application method can all be altered according to the response. Corticosteroids have an important role in skin diseases because of their anti-inflammatory [1], immunosuppressive [2], and anti-proliferative effects [3] on the keratinocytes.
The vasoconstrictor assay is the primary method for classifying the topical steroids according to their potencies [4].
The application of a steroid is followed by its absorption through the skin, during which time it must exert all its beneficial topical effects. The steroid diffuses into the stratum corneum, the outermost layer of the skin which not only serves as a barrier but also as a reservoir for topically applied substances. The reservoir function of the stratum corneum was first reported by Vickers in 1963. He demonstrated that a topically applied corticosteroid could exist as a reservoir in the stratum corneum for a few days, which was observed by the development of a physiological marker, a vasoconstriction [5].
This reservoir function of the skin was further studied by various workers by histo-autoradiography, by using radiolabelled drugs, chromametry and UV spectroscopy. By studying this reservoir effect of the topical corticosteroids in the skin, we can maximize the therapeutic benefits and the safety of the newer and the highly potent topical steroids.
Psoriasis is a chronic, inflammatory disease of the skin. Among the various dermatological diseases, it is a particularly well suited disease for evaluating the potency and the dosing frequency of the topical corticosteroids, since the disease sites are bilateral, symmetrical and of equal severity on both the sides [6]. The topical corticosteroids have improved the management of psoriasis, but these medications are also associated with certain adverse effects that are potentially serious.
Studies have shown that the once daily application of topical corticosteroids is as effective as the twice daily application. Continuous use of the topical clobetasol propionate cream, which is a superpotent steroid, can lead to various systemic and local adverse effects. Thus, in view of the reservoir effect, a very frequent application of a potent topical corticosteroid may not be necessary and it will only contribute to side effects, without any additional clinical benefit. Hence, it may be possible to further reduce the dosing frequency. By evaluating this reservoir effect of the corticosteroids, one can maximize their efficacy and safety as therapeutic agents. Therefore, there is need of more studies which compare the efficacy of different topical corticosteroids with the once daily regime versus the less frequent dosing.
We hypothesize that; even an alternate day application of a superpotent topical steroid like clobetasol propionate can be equally effective as the once daily application. The present study compared the once daily application of the clobetasol propionate 0.05% cream with the alternate day application in patients of a mild to moderate degree of plaque psoriasis, so that an application regimen could be devised, which would help in maximizing the efficacy and the safety of the topical corticosteroids.
MATERIALS AND METHODS
This study was carried out on the patients who attended the skin OPD in our hospital over a period of 1 year and 11 months. 89 patients with a mild to moderate degree of plaque psoriasis were found to fit the inclusion criteria and they were hence were selected for this study.
The approval of the institutional ethical committee was obtained and a written informed consent was taken from the patients.
Selection of the patients
Inclusion criteria
Patients of clinically diagnosed, mild to moderate plaque psoriasis (at least 10% of the body surface area involved)
Age group of 10-50 yrs
The patient is capable of reading, following instructions and understanding the application regimens (In minor patients, we ensured that this criteria were met in the parents)
The patient is committed to treatments and follow-up visits
Exclusion criteria
Extensive psoriasis which required systemic treatment.
The patient had taken the anti-psoriatic therapy within 3 weeks prior to the start of the study.
Drug induced psoriasis.
Pregnancy and lactation.
The patients who were already on systemic steroids for other reasons.
Any other debilitating/chronic illness.
METHODOLOGY
The enrolled patients completed a medical history form and an informed consent form, after which they were randomly assigned to two groups.
Group 1- Once daily application
Group 2- Alternate day application
The most prominent lesion was selected and the patients were prescribed the superpotent steroid – clobetasol propionate 0.05% cream (Tenovate cream®-GlaxoSmithKline). They were carefully instructed to apply a half Finger Tip Unit (FTU) [7] of the Tenovate cream on the selected patch either once daily or on alternate days according to their group allocation.
The initial few clobetasol propionate: Tenovate cream® (Glaxo- SmithKline) tubes were obtained as free samples, which were given by the medical representatives of the company. Later, we prescribed it on the patients’ OPD cards and ensured that the patients returned to show us the purchased tube, so that each patient took the drug of the same company and there was no variability because of the difference in the bioequivalence of the drugs of different companies. The patients of group 1 were instructed to apply half FTU once daily at the same time i.e in the morning. The patients of group 2 were instructed to apply half FTU on alternate days, also at the same time i.e in the morning. A compliance assessment was necessary, especially in the alternate day group, to ensure that they did not apply the cream every day. For this, the patients were asked to maintain a small dairy or a note pad, to tick mark the days of application and to bring it when they came for their next visit. We also asked the patients to bring the used/empty tubes, to judge whether they had strictly followed the instructions.
The patients were called for follow up in every 2 weeks gap i.e in the 2nd, 4th and the 6th weeks. We also took the phone numbers. of the patients who had one, so that in case if they did not come for the follow up visits, we could easily contact them and call them for their regular visits. Most of the patients turned up for their follow up visits.
The rescue medication was not given initially to any patient, because we selected only the mild to moderate cases of plaque psoriasis which had an approximate involvement of only 10% of the body surface area. But we carefully instructed all the patients to return or to report immediately if they suffered from any acute exacerbations or the Kobner’s phenomenon. Only 1 patient reported acute exacerbations of plaque psoriasis in group 2 after 1 week and she was prescribed Calciprotriol 50μg/gm tube twice a day for 1 week as a rescue medication. She was also given the option to exit the study but she preferred to continue with it.
The efficacy of the treatment was assessed by the Psoriasis Severity Index (PSI) which was recorded objectively according to the investigator’s assessment of erythema, induration and scaling at the baseline (0 week) and in the 2nd week, 4th week and the 6th week [8].
The Psoriasis Severity Index (PSI) was graded on a 0-4 scale. The score was defined as:
Grade 0: None/ Absent
Grade 1: Minimum - Light pink, Barely perceptible elevation, Rare scale.
Grade 2: Mild – Light red/pink, Slight elevation, Poorly defined scale.
Grade 3: Moderate – Red, Moderate elevation, Defined scales.
Grade 4: Severe – Dark Red, Marked ridge, Heavy scales
The PSI score was calculated as a sum of the erythema, induration and the scaling scores and it ranged between 0-12.
STATISTICAL ANALYSIS
The Student’s ‘t’ test was applied in the inter group comparison (once daily and on the alternate days) individually in the 2nd week, 4th week and the 6th week and ANOVA was applied in the intra group comparison by using the Origin software, version 6. A pvalue of < 0.05 indicated the difference to be significant.
RESULTS
Out of the total of the 89 patients who were recruited in the study, 1 dropped out each in group 1 and group 2 and 1 patient of group 2 was prescribed systemic corticosteroids.
Group 1- Once daily application (n=44)
Group 2- Alternate day application (n=42)
The mean age of the 44 patients in the once daily group was 28.5 ± 12.6 yrs. In the alternate day group, the mean age of the 42 patients was 27.8 ± 11.8 yrs [Table/Fig-1].
Demographic profile of patients in each group
| Groups | Mean Age(Yrs) | Sex |
| Male | Female |
| Once daily (n=44) | 28.5 ± 12.6 | 28(63.63%) | 16(36.36%) |
| Alternate Day (n=42) | 27.8 ± 11.8 | 27(64.28%) | 15(35.71%) |
There were 28 male (63.63%) and 16 female (36.36%) patients in the once daily group and 27 male (64.28%) and 15 female (35.71%) patients in the alternate day group [Table/Fig-1].
The localization of the selected plaques in the once daily group was – the upper limb-54.54%, the lower limb- 38.63% and the trunk- 6.81% [Table/Fig-2].
Localization of selected plaques in each group
| Groups | Localization of plaques |
| Upper Limb | Lower Limb | Trunk |
| Once daily (n=44) | 24(54.54%) | 17(38.63%) | 3(6.81%) |
| Alternate Day (n=42) | 20(47.61%) | 20(47.61%) | 2(4.76%) |
The localization of the selected plaques in the alternate day group was – the upper limb- 47.61%, the lower limb- 47.61% and the trunk- 4.76% [Table/Fig-2].
In the once daily group, the mean PSI score was reduced from 6.7 ± 1.37 at 0 weeks to 5.13 ± 1.45 in 2 weeks, to 3.45 ± 1.57 in 4 weeks and to 2.31 ± 1.49 in 6 weeks. The percentage reductions were- 24.22% in 2 weeks, 49.03% in 4 weeks and 65.87% in 6 weeks [Table/Fig-3].
Mean PSI scores & individual scores in once daily group (n=44)
| 0 Week | 2nd Week | 4th Week | 6th Week |
| Mean PSI | 6.77 ± 1.37* | 5.13 ± 1.45 | 3.45 ± 1.57 | 2.31 ± 1.49 |
| Erythema | 2.47 ± 0.84 | 2 ± 0.83 | 1.47 ± 0.82 | 1 ± 0.80 |
| Induration | 1.79 ± 0.66 | 1.34 ± 0.68 | 0.81 ± 0.69 | 0.52 ± 0.62 |
| Scaling | 2.5 ± 0.66 | 1.79 ± 0.79 | 1.15 ± 0.74 | 0.79 ± 0.73 |
In the alternate day group, the mean PSI score was reduced from 6.4 ± 1.46 at 0 week to 5.45 ± 1.31 in 2 weeks, to 4.45 ± 1.29 in 4 weeks and to 3.11 ± 1.51 in 6 weeks. The percentage reductions were- 14.84% in 2 weeks, 30.46% in 4 weeks and 51.4% in 6 weeks [Table/Fig-4].
Mean PSI scores & individual scores in alternate daily group (n=42)
| 0 Week | 2nd Week | 4th Week | 6th Week |
| Mean PSI | 6.40 ± 1.46* | 5.45 ± 1.31 | 4.45 ± 1.29 | 3.11 ± 1.51 |
| Erythema | 2.52 ±0.86 | 2.16 ± 0.69 | 1.83 ± 0.65 | 1.33 ± 0.81 |
| Induration | 1.57 ± 0.83 | 1.5 ± 0.80 | 1.28 ± 0.80 | 0.81 ± 0.80 |
| Scaling | 2.33 ± 0.84 | 1.78 ± 0.84 | 1.33 ± 0.72 | 0.97 ± 0.84 |
The erythema score was reduced from 2.47 ± 0.84 to 1 ± 0.80 i.e there was a 59.52% reduction in 6 weeks in the once daily group [Table/Fig-3]. In the alternate day group, it was reduced from 2.52 ± 0.86 to 1.33 ± 0.81 i.e there was a 78.96% reduction in 6 weeks [Table/Fig-4].
The induration score was reduced from 1.79 ± 0.66 to 0.52 ± 0.62 i.e there was a 70.95% reduction in 6 weeks in the once daily group [[Table/Fig-3]. In the alternate day group, it was reduced from 1.57 ± 0.83 to 0.81 ± 0.80 i.e there was a 48.41% reduction in 6 weeks [Table/Fig-4].
The scaling score was reduced from 2.5 ± 0.66 to 0.79 ± 0.73 i.e there was a 68.4% reduction in 6 weeks in the once daily group [Table/Fig-3]. In the alternate day group, it was reduced from 2.33 ± 0.84 to 0.97 ± 0.84 i.e there was a 58.37% reduction in 6 weeks [Table/Fig-4].
A complete resolution was seen in 2 (4.54%) patients in the once daily group after 6 weeks, but no patients (0%) showed a complete resolution in the alternate day group.
The intra group comparison of the mean PSI score and the erythema, induration and the scaling scores in the once daily and the alternate day groups by ANOVA demonstrated that all the F values were statistically significant for each parameter (P < 0.05), thus indicating the significant efficacy of the clobetasol propionate cream in the patients of mild to moderate plaque psoriasis individually in both the groups [Table/Fig-5].
Intra group comparison of mean PSI & individual scores in both groups
| Groups | Once Daily (n=44) *F (P Values) | Alternate Day (n=42) *F (P Values) |
| Mean PSI | 76.60 (P < 0.05). | 42.40 (P < 0.05). |
| Erythema | 26.25 (P < 0.05). | 18.49 (P < 0.05). |
| Induration | 31.39 (P < 0.05). | 7.54 (P < 0.05). |
| Scaling | 45.58 (P < 0.05). | 21.80 (P < 0.05). |
The inter group comparisons on each individual visit i.e in the 2nd, 4th and the 6th weeks by the Student ‘t’ test showed that in the 2nd week, there was no statistically significant difference in the efficacy of the treatment (P > 0.05) in the once daily and the alternate day groups. But in the 4th and the 6th weeks, there was a significant difference in the treatment (P < 0.05). This showed that the efficacy of the once daily and the alternate day groups was the same in the 2nd week, but it was significantly lower in the alternate day group in the 4th and 6th weeks [Table/Fig-6 & 7].
Comparison of Mean PSI score in once daily (n=44) and alternate day (n=42) group on each visit
| 2nd Week | 4th Week | 6th Week |
| T value | P value* | T value | P value* | T value | P value* |
| 1.05621 | 0.2939 | 3.20179 | 0.00193 | 2.46877 | 0.01558 |
Graph showing Mean PSI score in once daily and alternate day groups
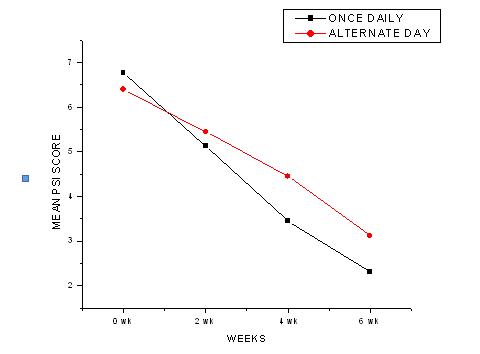
Therefore, it can be concluded that the alternate day application, though it may be effective initially, its efficacy decreases later.
DISCUSSION
There is an extensive use of topical steroids in non-infective and in- flammatory dermatoses. Newer topical corticosteroids have been introduced in the recent years, which are highly potent. These newer molecules have a higher anti-inflammatory effect and good compliance among the patients, but have more adverse effects. The choice of a dermato-corticosteroid depends on the suspected sensitivity of the disease, its degree of extension, its anatomical site, as well as the patient’s age and the planned duration and the frequency of the treatment. In general, the acute inflammatory eruptions respond well to the mild/moderate strength topical steroids. The chronic, thickened or hyperkeratotic dermatoses may require potent or super potent steroids. The less responsive the disease is, the greater is the potency of the corticosteroid that may be required.
Psoriasis is a chronic non infectious, inflammatory disease of the skin which is characterized by well defined erythematous plaques with large, adherent, silvery scales. The common areas which are affected are the extensor aspects of the extremities, particularly the elbows, knees, the sacral regions of the back and the scalp. The lesions may be asymptomatic but some patients may have severe itching. The disease may show spontaneous remissions and relapses at variable intervals, but most of the patients get worse during the winters. It is generally not a fatal disease, but it can cause significant morbidity, social embarrassment, financial loss and disruption in the patients’ lives.
The main defect in psoriasis is the mechanism which controls the rate of epidermal cell division. Normally, the epidermal cells divide at a fixed control rate and the newly formed cells at the basal layer take 28 days to transform and gets shed off from the skin surface as keratinized cells. In psoriasis, there is increased epidermal proliferation due to the excessive division of the cells in the basal layer. The transit time of the keratinocytes through the epidermis is shortened and the epidermal turnover time falls from 28 days to 4- 5 days. Hence, the immature cells are shed as silvery white scales. Though the aetiology is unknown, certain factors which are involved in its pathogenesis are known. It has a genetic predisposition i.e. an autosomal dominant transmission with irregular penetrance. Biochemical abnormalities which are caused by the increased levels of prostaglandins, leukotrienes and hydroxyeicosatetraenoic acids are involved. Certain interleukins like IL-1, IL-2, IL-6, IL-8 and IFN- γ and growth factors- likeTNF- α are elevated.
The treatment of psoriasis includes topical preparations like corticosteroids, keratolytics, anthralin, coal tar and retinoids, phototherapy and systemic and biological medications such as immunosuppressant and monoclonal antibodies, or their combinations.
Clobetasol propionate is the most potent of the currently available topical steroids, as it has been predicted by the vasoconstrictor assay. It belongs to the group of superpotent (class I) steroids according to American classification [9]. It has proved to be more effective in psoriasis, apart from other steroid responsive dermatoses. It prolongs the remission rates, so that intermittent treatment schedules are possible with minimum side effects [10].
The pharmacokinetics of the topical drugs describes the time dependent passage of the drugs out of the vehicle or the device which is applied on to the skin surface and subsequently through the skin barrier into the underlying layers and into the systemic circulation, with some of the compounds being metabolized and excreted during this passage. The viable epidermis below the stratum corneum is metabolically active and it contains the cytochrome P450 enzyme, which causes hydrolysis or sulfate conjugation of most of the topically applied steroids within the epidermis, but since clobetasol is a fluorinated steroid and as it also contains a substituted 17-hydroxyl group, it is not metabolized in the skin and is retained in the stratum corneum as a reservoir or depot, which further increases its clinical efficacy.
The side effects of the topical steroids may originate from their unnecessarily high drug concentrations, their frequent applications and the long term treatment with a highly potent steroid. A number of studies have shown that even a short-term exposure to potent corticosteroids can exert profound negative effects on the cutaneous structure and function. Clobetasol propionate is a highly potent topical corticosteroid that has been shown to suppress the HPA axis at low doses also. The systemic absorption of the topical corticosteroids has resulted in a reversible HPA axis suppression [11], stunting of growth in children, manifestations of Cushing’s syndrome, hypertension, hyperglycaemia, and glycosuria in some patients. The local side effects include epidermal thinning, melanocyte inhibition, dermal striae, skin atrophy, telangiectasia, purpura and other vascular side effects. The regular and the frequent usage of the higher potency steroids also leads to tachyphylaxis [12], and their abrupt discontinuation can cause a rebound dermatological condition. Therefore, the clinicians have to be very cautious while they prescribe the higher potency topical steroids.
There is also a reservoir function of the stratum corneum which was first reported by Vickers in 1963 [5]. After Vickers, many researchers established this reservoir function of the stratum corneum but the recent studies which confirmed the reservoir effect of the topical corticosteroids were those which were done by Hayakawa et al., Pelchrzim et al., Teichmann et al., and Abidi et al., By studying this reservoir effect of the topical corticosteroids on the skin, we can explain why a lesser application frequency can be effective for the dermatoses with a normal keratin layer. Abidi et al., demonstrated the reservoir of the topical corticosteroid, clobetasol propionate 0.05% cream, (Class I Superpotent- according to the American classification) in rabbit skin upto 4 days by the histamine induced wheal suppression test [13]. Since the rabbit skin is similar to human skin, therefore, this reservoir effect can lead to a prolonged action of the topical clobetasol propionate 0.05% cream even in humans. Therefore, dermatologists should prescribe these high potency steroids less frequently and very cautiously with clear instructions for their usage. This will decrease the amounts of the steroids which are used and decrease their costs as well as the local and the systemic adverse effects and also the phenomenon of tachyphylaxis which occurs with the continuous use of topical steroids.
Since decades, there has been a debate on the corticosteroid application frequency, so as to obtain the maximum benefit with minimum side effects. Few studies have proved the efficacy of the once daily application in various dermatological diseases.
Bleehen et al., showed that the once daily treatment with the fluticasone propionate 0.05% cream in atopic eczema was as effective as the twice daily treatment [14].
Singh et al., in 1995, evaluated the efficacy of topical 0.05% betamethasone dipropionate in psoriasis and concluded that the once a day application was as effective as the twice a day application [15].
According to the study of Narasimha, Srinivas and Mathew, the once-daily application of the clobetasol propionate (0.05%) cream was as effective as the twice-daily application, but the alternateday application was less effective than the once-daily application, which was assessed by doing the histamine-induced wheal suppression test on the human skin [16].
Another study of Singh S showed that the once daily application of the topical steroids was as effective as the twice daily application in psoriasis [17].
Singh, Singh, and Pandey, in 1998, evaluated the effect of the duration of the application and the dosing frequency of the topical 0.1% mometasone furoate ointment in psoriasis by the LPSI score and concluded that increasing the dosing frequency from once to twice daily does not increase the efficacy of the mometasone furoate ointment in psoriasis [18].
In another open multi-centre trial which was done by Svartholm , Larsson and Frederiksen, 316 psoriasis patients were treated with the clobetasol propionate intermittently over a 14-day period. The treatment provided rapid healing of the psoriasis patch in 62% of the patients [19].
English, Bunker, Ruthven et al., in 1989 also confirmed the ef- ficacy of the once daily betamethasone dipropionate cream versus its twice daily application in the treatment of steroid sensitive dermatoses [20].
In reference to these studies and in view of the reservoir effect of the topical corticosteroids, it may be possible to reduce the dosing frequency further, while preserving the therapeutic effi- cacy. The frequencies of application of the topical steroids have remained an unresolved issue since their introduction. Therefore, there is need of studies which compare the efficacy of the different topical corticosteroids with the once daily regime versus a less frequent dosing. Several ADRs which are associated with the use of the topical corticosteroids are likely to be ameliorated with their less frequent use [21], especially skin atrophy, which was reduced when the topical steroids were applied at relatively longer intervals [22].
We hypothesized that the increased frequency of application of the topical corticosteroids after their saturation was unlikely to be of a therapeutic benefit and that it may only contribute to the side effects of the steroids. The goal of the treatment was to achieve the maximum desired effects and minimum side effects.
The present study compared the once daily application of the clobetasol propionate 0.05% cream (Tenovate cream®) with the alternate day application in patients with a mild to moderate degree of plaque psoriasis. The clinical observations were made objectively on the basis of the PSI score, which was calculated according to the severity of the erythema, induration and the scaling.
The intra group comparison of the mean PSI score in the once daily and the alternate day groups by ANOVA showed the means to be significantly different in each group (P < 0.05), thereby demonstrating that each regimen was effective individually, but on comparing the PSI scores of the once daily and the alternate day groups on each visit by the Student ‘t’ test, it was seen that in the 2nd week, there was no statistical difference in the efficacy of the treatment (P>0.05) but that in the 4th and 6th weeks, there was a significant difference in the treatment (P<0.05). This showed that the efficacy of the once daily and the alternate day groups was the same in the 2nd week, but that it was signifi- cantly lower in the alternate day group in the 4th and 6th weeks (P>0.05). Clinically, the patients in the once daily group showed better improvement of the selected lesion at 6 weeks.
Therefore, it can be concluded that the alternate day application, though it may be effective initially, its efficacy decreases later. This decreased efficacy may be due to the increased turn over rate of the keratinocytes in psoriasis and thus the immature cells may not be able to store the steroid which is applied topically.
This study can help us to device a regime which will decrease the total amount of the topical corticosteroid which is applied, without compromising on the efficacy. In mild dermatoses which require a short period of treatment, we can prescribe the highly potent topical corticosteroids like clobetasol propionate only after the less potent steroids have become ineffective. This can be applied in the alternate day regimen, as its efficacy is at par with that of the once daily application, thus avoiding unnecessary exposure and the local and systemic side effects, decreasing the development of tolerance, reducing the cost factor and improving the overall patient compliance.
But in the cases of moderate to severe dermatoses which require a long treatment with a potent steroid, initially we can recommend the alternate day application for 14 days, followed by the once daily application, as its efficacy is better than that of the alternate day regimen after 2 weeks.
CONCLUSION
This study showed that the efficacy of the once daily and the alternate day group application of the clobetasol propionate 0.05% cream (Tenovate cream®) in patients with a mild to moderate degree of plaque psoriasis was the same in the 2nd week, as there was no statistically significant difference in the PSI scores (P>0.05), but it was significantly lower in the alternate day group at the 4th and 6th weeks (P<0.05). Clinically, the patients in the once daily group showed better improvement of the treated lesion in the 6th week, though individually, each regimen was effective.
Hence, the alternate day application of the topical steroid, the clobetasol propionate cream may be effective as a once daily application initially in patients who have mild to moderate plaque psoriasis, but later its efficacy decreases.