A Survey on the Knowledge, Attitude and the Practice of Pharmacovigilance Among the Health Care Professionals in a Teaching Hospital in Northern India
Hardeep1, Jagminder Kaur Bajaj2, Kumar Rakesh3
1 Tutor, Department of Pharmacology, PIMS, Jalandhar, India.
2 Professor and Head, Department of Pharmacology, PIMS, Jalandhar, India.
3 Assistant Professor, Department of Pharmacology, PIMS, Jalandhar, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Jagminder Kaur Bajaj, Professor and Head, Department of Pharmacology, PIMS, Jalandhar, India.
Phone: 9417876566, 9041317739
E-mail: pharmaco@pimsj.com
Objective: To assess the pharmacovigilance awareness among the healthcare professionals in a teaching hospital in Northern India.
Material and Methods: A questionnaire which was suitable for assessing the basic Knowledge, Attitude and the Practice (KAP) of pharmacovigilance was designed and distributed among 100 doctors of the Punjab Institute of Medical Sciences (PIMS) Hospital, Jalandhar, Punjab, India.
Results: Among the 100 doctors, 61 responded. The data was analyzed by using the SPSS statistical software. Although 77% of the subjects knew the term ‘pharmacovigilance’, only 59% were aware of the existence of the National Pharmacovigilance Program. 23% volunteered to reports Adverse Drug Reactions (ADRs), but more than 60% doctors did not know how and where to report the ADRs.
Conclusion: There is a need for a regular training and the reenforcement for the ADR reporting among the health care personnel. The perception of the reporting process being tedious, the lack of time, a poor knowledge on the reporting mechanism and inadequate expertise seemed to be the main reasons for not reporting the ADRs. A majority of the respondents suggested regular training sessions on a priority basis for the success of the pharmacovigilance program and for the better clinical management of the patients in general.
Pharmacovigilance, Knowledge, Attitude, Practice, Adverse drug reactions
INTRODUCTION
Adverse Drug Reactions (ADRs) are an important cause of morbidity and mortality worldwide [1,2]. According to World Health Organization (WHO) definition, an ADR is any noxious, unintended, and undesired effect of a drug, which occurs at the doses which are used in humans for prophylaxis, diagnosis, or therapy. ADRs are a threat to the patient’s safety and the quality of life and they increase the health care cost considerably. In 1994, the healthcare costs which were caused by ADRs were 4 billion dollars. In a report which was published by the FDA in 1989, 12000 cases of death were caused by ADRs. So, a proper monitoring for the prevention and the management of ADRs is need of the hour.
Pharmacovigilance is, “The science and the activities which relate to the detection, assessment, understanding and the prevention of adverse effects or any other drug-related problems” [3]. The Uppsala Monitoring Centre (UMC, WHO), Sweden, maintains the international database of the adverse drug reaction reports. It has been estimated that only 6-10% of all the ADRs are reported [4].
Although, India is participating in the program, its contribution to the UMC database is very little. This is essentially due to the absence of a vibrant ADR monitoring system and also due to a lack of the reporting culture among the health care workers [5]. In order to improve the reporting rate, it is important to improve the Knowledge, Attitude and the Practices (KAP) of the healthcare professionals with regards to the ADR reporting and the pharmacovigilance. This study was a step which was taken in that direction and it endeavoured at evaluating the baseline KAP the doctors at a teaching hospital, regarding the ADR monitoring and pharmacovigilance.
MATERIALS AND METHODS
This was a cross sectional, questionnaire based survey which was conducted in a tertiary care hospital (Punjab Institute of Medical Sciences, Jalandhar) in the state of Punjab (India). It was conducted on doctors from the clinical, paraclinical and the preclinical fields. The study instrument was a pre designed questionnaire which was structured to obtain information on the knowledge of the ADRs reporting, the attitudes towards the reporting, and the factors that in practice could hinder the reporting among the doctors. Suggestions on the possible ways to improve the ADR reporting were welcome. The doctors were requested to complete the questionnaire and to return it within 1 day to their respective departmental offices.
RESULTS
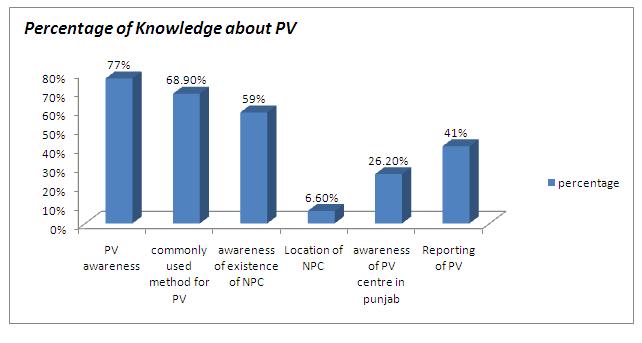
Among the 100 circulated questionnaires, 61 which were duly filled were received back. The questionnaire was divided into three sections of knowledge (6 questions), attitude (3 questions) and practice (one question). All the calculations were done by using the SPSS software. [Table/Fig-1 & 2] shows the knowledge of the study group with respect to pharmacovigilance. 77% subjects responded right regarding the definition of pharmacovigilance. 68.9% of the subjects knew about “post marketing surveillance” as a method which was employed by the pharmaceutical companies for monitoring the ADRs of the newly launched drugs. 59% subjects were aware of the existence of a national pharmacovigilance centre in India, but only 6.6% subjects knew where it was located. Only 26.2% subjects responded correctly about the location of the peripheral pharmacovigilance centre in Punjab, India. 41% subjects felt that the ADRs reporting should be sent to a regulatory body within 7 days.
Knowledge of pharmacovigilance
| S.No. | Most frequent answer | Right answer | Wrong answer |
| 1. Pharmacovigilance deal with | ADRs (77%) | ADRs (77%) | 33% |
| 2. which method is commonly employed by pharmaceutical companies for pharmacovigilance of new drugs once they are launched in the market | Post marketing surveillance (68.9%) | Post marketing surveillance (68.9%) | 31.1% |
| 3. Are you aware of existence of NPC in India | Yes (59%) | Yes (59%) | 41% |
| 4. If yes, then where is it located | AIIMS, Delhi (34.4%) | CDSCO (6.6%) | 93.4% |
| 5. Are you aware of existence of pharmacovigilance centre in Punjab? If yes, where | Not known (59%) | PGIMER (26.2%) | 73.8% |
| 6. In India, pharmacovigilance reporting should be sent to regulatory body within | 7 days (41%) | 7 days (41%) | 59% |
Percentage of Knowledge about PV
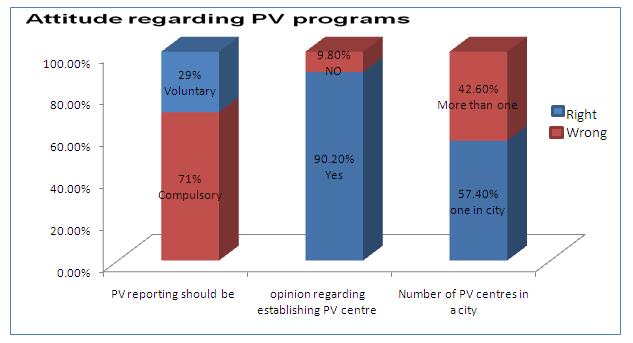
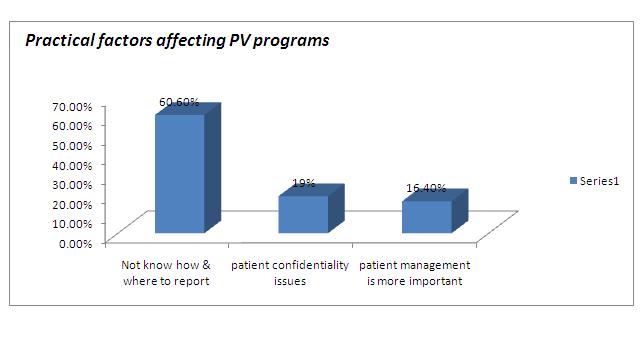
[Table/Fig-3 & 4] shows the attitude of the study group towards pharmacovigilance. Only 23 % subjects had a willingness to report the ADRs voluntarily. Most of subjects (90.2%) agreed to the proposal of the establishment of pharmacovigilance monitoring centre in their working institutions, but 57.4% felt that one such centre in a city was sufficient. The most common practical difficulty which was faced by the doctors in the reporting of ADRs was that a majority of them (60.6%) did not know how and where the ADRs had to be reported [Table/Fig-5 & 6]. Hence, most of them suggested that pharmacovigilance awareness programs should be organized as seminars or workshops.
Attitude towards pharmacovigilance
| S.No. | Most frequent answer | Right answer | Wrong answer |
| 1.Pharmacovigilance reporting should be | Compulsory (70.5%) | Voluntary (23%) | 77% |
| 2.What is your opinion about establishing pharmacovigilance monitoring centre in your institution | Yes (90.2%) | Yes (90.2%) | 9.8% |
| 3. How many pharmacovigilance centers should be there in a city | One in a city is sufficient (57.4%) | One in a city is sufficient (57.4%) | 42.6% |
Attitude regarding PV programs
Practical factors in pharmacovigilance
| S.No. | Not knowing how & where to report | Patient confidentiality issues | Managing patient is more important |
| 1. What are the factors discourage you from taking part in pharmacovigilance programs | 60.6% | 19% | 16.4% |
Practical factors affecting PV programs
The relationship between the knowledge and the attitudes of the doctors was investigated by using Pearson’s correlation coefficient. Preliminary analyses were performed to ensure that there was no violation of the assumption of the normality, linearity and the homosctdasticity. [Table/Fig-7] shows that there was a medium, positive correlation between the knowledge and the attitudes of the doctors (r= 0.316, n=61, p<0.013).
| knowledge | Attitude |
| knowledge Pearson | 1 | 316* |
| Correlation | | .013 |
| Sig. (2-tailed) N | 61 | 61 |
| attitude | 316* | 1 |
| Pearson Correlation | .013 | |
| Sig. (2-tailed) N | 61 | 61 |
DISCUSSION
This study showed little awareness about the ADR reporting system among the doctors in a tertiary care hospital. This was in agreement with the results of Li Qing et al., [6]. The doctors did not know where and how the ADRs had to be reported, the time limits for the reporting, etc. Therefore, it seems necessary to hold awareness programmes to improve the ADR reporting. The paramedical staff should also be encouraged for the ADRs reporting, since they are in closer contact with the patients for a longer duration and as they can play an important role in making the pharmacovigilance programs more efficacious. A majority of the doctors (71 %) felt that the ADR reporting should be compulsory, which again matched with the results which were obtained by Li Qing et al., and Karen J Belton et al., but not with those which were obtained by Bateman et al., [6–8]. Very few doctors wanted to report an event if it was an already well recognized adverse reaction. In the clinical practice, the factors that discourage the doctors from a spontaneous reporting are a lack of knowledge about the reporting procedure (60.6%) and other practical issues such as the patient management (16.4%) and the patient confi- dentiality issues (19%).
A majority of the doctors opined that the ADR reporting should be compulsory (71 %) and some felt that it should be voluntary (29%). To improve the spontaneity in the reporting rates, the doctors suggested the organization of training programmes and an uncomplicated reporting system with a quick feedback regarding their specific reports. A similar study which was done by Manuela Tabali et al., demonstrated that an educational intervention could increase the physicians’ awareness on ADRs and that the physicians would be able to incorporate the knowledge that they gained from their training into their everyday clinical practice [9]. This had to be reinforced by holding regular seminars/workshops, etc. In order to generalize our findings, it is imperative that similar studies be done in other teaching hospitals of the country. In conclusion, our study strongly suggested that there was a great need to create awareness among the doctors to improve the reporting of ADRs. The training sessions must clarify the roles of the various healthcare professionals in pharmacovigilance. There should be closer relationship between the doctors and the pharmacovigilance centres. The ADR reporting should be made an integral part of the clinical activities in order to improve the patient care QUESTIONAIRE.
[1]. Lazarou J, Pomeranz BH, Corey PN, The incidence of adverse drug reactions in hospitalized patients-a meta- analysis of prospective studiesJAMA 1998 279:1200-05.Doi:10.1001/jama.279.15.1200. Available from: http://jama.amaassn.org/cgi/content/full/279/15/1200 [Google Scholar]
[2]. Classen DC, Pestotnik SL, Evans RS, The adverse drug events in hospitalized patientsJAMA 1997 277(4):301-06.PMID: 9002492. Available from: http://www.ncbi.nlm.nih.gov/pubmed/9002492 [Google Scholar]
[3]. The World Health OrganizationSafety of medicines: A guide to detecting and reporting adverse drug reactions 2002 GenevaWHO/EDM/QSM/2002 2 [Google Scholar]
[4]. Feely J, Moriarty S, O’Connor P, Stimulating the reporting of an adverse drug reaction by using a feeBr Med J 1990 300:22-23.Available from: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC1661889/pdf/bmj00160-0028.pdf [Google Scholar]
[5]. Inman WH, The attitudes to the adverse drug-reaction reportingBr J Clin Pharmacol 1996 41:433-435.Available from: http://www.ncbi.nlm.nih.gov/pubmed/8735689 [Google Scholar]
[6]. Qing L, Su-min Z, Hua-ting C, Shi-ping F, Xin Y, Dong L, Z.,: The awareness and the attitudes of the healthcare professionals in Wuhan, China about/towards the reporting of adverse drug reactionsChinese Medical Journal 2004 117(6):856-61.Available at: http://www.cmj.org/periodical/PaperList.asp?id=LW7205 [Google Scholar]
[7]. Belton KJ, Lewis SC, Payne S, Rawlins MD, Wood SM, An attitudinal survey on the adverse drug reaction reporting by the medical practitioners in the United KingdomBr J Clin Pharmacol 1995 39(3):223-26.Available from: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC1364995 [Google Scholar]
[8]. Bateman DN, Sanders GLS, Rawlins MD, The attitudes to the adverse drug reaction reporting in the northern regionBr. J. Clin. Pharmacol. 1992 34(5):421-26.Available from: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC1381471/?tool=pubmed [Google Scholar]
[9]. Tabali M, Jeschke E, Bockelbrink A, Witt CM, Willich SN, Ostermann T, Matthes H, An educational intervention to improve the physician reporting of the adverse drug reactions (ADRs) in a primary care setting in the complementary and alternative medicine programsBMC Public Health 2009 9:274Available at: http://www.biomedcentral.com/1471-2458/9/274 [Google Scholar]