Introduction: Anabolic steroid is an established drug for treating catabolic states. The said drug has been shown to restore the lean body mass, to prevent osteoporosis and to correct the impaired immune response, but only few studies have evaluated the effect of the same on fracture healing.
Methods: Fracture was produced by an impact device in 24 rabbits which were divided into experimental (I) and control (II) groups of 12 animals each. The experimental groups were further divided into the subgroups IA and IB of equal animals, which received Nandrolone Decanoate 10 mg/kg intramuscularly, biweekly for 2 weeks and 4 weeks respectively.
Results and Conclusion: Radiographs taken on the post fracture day15 and 40 showed better healing in the Nandrolone Decanoate administrated groups as a dense periosteal bone formation and prevention of the local osteoporosis. Histochemical examination of the callus and high serum alkaline phosphatase levels on day 15 and 40 confirmed better mineralization in experimental animals.
Anabolic steroid, Fracture healing, Tibial fracture model
INTRODUCTION
Fracture healing is a specialized repair process which involves a well-characterized cascade of events, which includes three major stages i.e. –inflammation reaction, callus formation and callus remodeling [1–4]. The functional recovery after a fracture is often incomplete, especially in the older ages. A catabolic state develops after fracture and following surgery, which contributes to a poor outcome [5–9].
Many patients cannot return to a normal life, which causes a serious impact on their socio-economic status. [10–12]. The patients who sustain fractures, especially those which involve the lower limb, lose body-weight after surgery. This situation leads to generalized weakness, impaired immune response and slower wound and fracture healing [13,14].
Anabolic steroid produces a positive nitrogen and calcium balance, and it is an established drug for treating catabolic states. The effect of an anabolic steroid in the patients who sustain fractures and in animal models, has been evaluated sparingly, although it has been shown to restore the lean body mass, to prevent osteoporosis and to correct the impaired immune response [15,16].
A fracture was created at the mid-diaphysis of the tibia in vivo, for monitoring the bone healing in rabbit. The complete disruption of the cortex and the medulla by an impact force in a pre-drilled tibial shaft is technically simple and is a highly reproducible method for creating a bone defect for normal rabbit fracture healing study. This method does not disturb the normal walking activities of the rabbit [17,18]. Clinically, bridging of the fracture site with cortices or a cortical continuity is the most commonly reported criteria for a radiological assessment of the fracture union at any injury location [19].
In the present study, we examined the differences in the cortical bone healing over a time period of 6 weeks between the control rabbits and the rabbits which received an anabolic steroid. A radiograph was employed for the analysis of the callus mineralization at different time intervals. Decalcified histology was used to describe the callus histopathology features at the tissue level, along with the calcium in the callus, to assess the mineralization activity. Histochemical analysis and serum alkaline phosphatase estimation were done to evaluate the systemic and the local bone turnover.
MATERIALS AND METHODS
Experimental Animals
Twenty four albino rabbits (weight: 1.5-2 kg) were obtained from the animal house of J.N. Medical College and they were housed under standard conditions. They were kept on pellet diet (Lipton India limited) and water ad-libitum. The institutional animal ethics committee of the medical college approved the experimental protocol of the study (Registration No. 401/CPCSEA; Dated: 30.03.2010).
Surgical Protocol and experimental design
Rabbits were divided into experimental (I) and control (II) groups of 12 animals each and fractures were produced by using an impact device by the Hiltunen method [20]. The rabbits were anaesthesized by giving thiopentone sodium, 30-50 mg/kg, intraperitoneally. A 1.5 mm Kirschner wire was drilled into the tibial shaft percutaneously, through the tibial tuberosity. The pre-nailed tibial shaft was fractured by an impact device, which resulted in a transverse fracture, which was confirmed by a radiograph. A K-wire was used to get an appropriate alignment and stabilization and to prevent comminution at the fracture site. Group I received injection Nandrolone decanoate (anabolic steroid) intra-muscularly and the control animals of Group II received equal volumes of the vehicle only. The Group I rabbits were further subdivided into the subgroups IA and IB of six animals each. Subgroup IA animals received Nandrolone Decanoate 10mg/kg intra-muscularly, biweekly for 2 weeks and the subgroup IB rabbits received Nanadrolone decanoate 10mg/kg biweekly for 4 weeks.
Radiographs were taken on day 0, day 15 and day 40 to assess the fracture healing. The serum alkaline phosphatase was also estimated on the aforementioned days. After 40 days, the animals were sacrificed and sections were fixed in 10% formalin. Callus was obtained from the fracture sites for doing histochemical and histopathological analyses.
STATISTICAL ANALYSIS
Student’s t-test was used for the parametric data, to compare the control and the experimental groups. A p value of <0.05 was considered to be statistically significant.
RESULTS
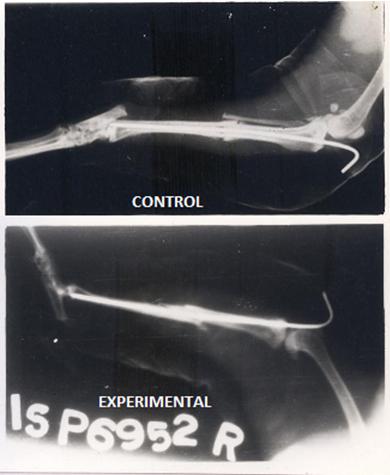
No significant differences in the body weight or the appetite were noticed throughout the period of the study. The experimental rabbits showed better healing in the form of dense calluses and disappearance of the gap between the fragments by the formation of a cortical as well as a medullary bridging as compared to the control animals, which showed only cortical bridging [Table/Fig-1].
Radiographs of fracture tibia in control and experimental animal showing minimum gap and very dense bone formation at fracture site in experimental animal treated with Nandrolone (At 6th week).compared to control
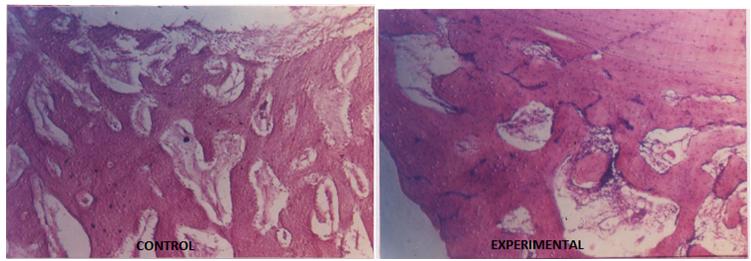
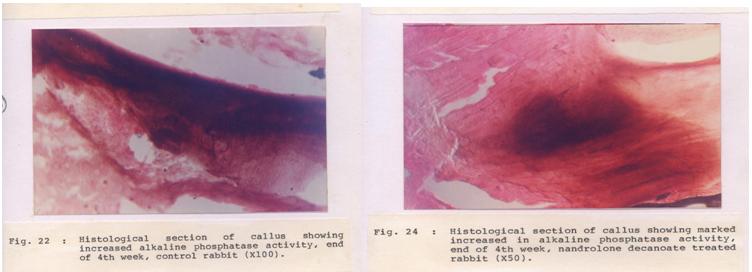
Histopathological observation showed very thick trabeculae and the formation of bony lamellae, which led to consolidation in both the groups. But collagen activities and osteoblastic proliferation were significantly better in the experimental group [Table/Fig-2]. A high osteoblastic activity was also confirmed by the histochemical examination of the callus and a high serum alkaline phosphatase level on day15 [Table/Fig-3] in the experimental group [Table/Fig-4]. The mean calcium concentrations in the callus in the control group were 0.89±0.01 and 1.04±0.41 mg/g on days 15 and day 40 respectively.
Section of callus: well developed compact bone formation in experimental animal as compared to control animal. (H&E stain, 50X)
Section of callus: High alkaline phosphatase activity in experimental animals. (50X) compared to control
Effect of Nandrolone decanoate (10 mg /kg IM) on serum alkaline phosphatase level
| Serum alkaline phosphatase (K.A.U./ 100 ml) , mean ± SE |
| Period | Control | Experimental | % change | P* <0. 001 Highly significant |
| 2 weeks | 18.17±0.75 | 25.0±0.58* | + 37.59 |
| 6 weeks | 13.50± 0.43 | 19.83±0.45* | + 46.88 |
On the other hand, a significantly better mineralization was observed in the experimental group (1.08±0.05 mg/g on day 15 and 1.21±0.06 mg/g on day 40) [Table/Fig-5]. After 40 days, there was significant fall in the serum alkaline phosphatase level in both the groups, but the level of the fall was significantly higher in the experimental group [Table/Fig-6].
Effect of Nandrolone decanoate (10 mg /kg IM) on calcium at fracture site.
| Calcium (mg/g) mean ± SE |
| Period | Control | Experimental | Percent change | P* <0. 01 P** <0. 05 significant |
| 2 weeks | 0.89±0.01 | 1.08±0.05* | + 21.35 |
| 6 weeks | 1.04±0.41 | 1.21±0.06** | + 16.35 |
Effect of Nandrolone decanoate (10 mg /kg IM) on calcium at fracture site.
| Serum alkaline phosphatase (K.A.U./100 ml) , mean ± SE |
| Period | 2nd week | 6th week (40th day) | Percent change | P* <0 . 01 P** <0. 001 |
| Control | 18.17±0.75 | 10.62±0.23* | - 41.55 |
| Experimental | 25.0±0.58 | 6.13±0.87** | -75.48 |
DISCUSSION
Anabolic steroid is an established drug for the acute catabolic states and the osteoporosis in elderly males. But its role in fracture healing still is inconclusive and no data is available till date for comparison. The Nandrolone decanoate administered groups showed better fracture healing as a dense periosteal bone formation and prevention of the local osteoporosis. This evidence was reported previously by Yi-Xin He et al., (2011), who had studied the bone healing pattern in mice by inducing ovariectomy-induced osteoporosis [21]. This fact was also confirmed by Aerssens et al., (1993), who observed the affect of oestrogen and progesterone indirectly by performing ovariectomies in female rats [22]. Several other scientists also advocated that anabolic steroids produced a better bone mass density and that they prevented osteoporosis [23–26].
The osteoblastic activities were very high in the Nandrolone administered animals than in the control group. The osteoblastic activity was assessed by measuring the serum alkaline phosphatase levels and locally in the callus by measuring the alkaline phosphatase activity, by Nilsson and Granstrom (1987). The high levels of serum alkaline phosphatase and the callus alkaline phosphatase activity in the experimental group in the present study demonstrated a better fracture healing than that in the control group [27].
Frankle and Borrelli (1990) reported high levels of calcium in the healing callus with the use of anabolic steroids. We observed that the Nandrolone treated animals achieved higher levels of callus calcium than the control group, which showed a better bone mineralization activity [28].
Anabolic steroid on plasma hydroxyproline (HOP) was investigated in young male rabbits, following operative fractures of the radius. The plasma HOP was found to increase during the fracture healing in the control animals, particularly in the first week and during the callus remodelling. The animals which were treated with the anabolic steroid did not present the initial rise, but they presented a sustained increase during the callus remodelling [29]. The administration of an anabolic steroid (17α-methyl-17β- hydroxy-androsta-1,4-dien-3-one) in rats improved the tensile strength of the experimental granulation tissue and of the healing skin wounds on the 12th postoperative day. This, however, occurred only in the undernourished animals which showed a retarded development of the connective tissue. This difference was also reflected in the collagen content of the granulation tissue. The treatment caused a slight increase in the tensile strength in all the feeding groups on the second postoperative day [30]. In a prospective randomized trial, a protein-rich liquid supplementation in combination with an anabolic steroid, which was given for 6 months to lean elderly women with femoral neck fractures, was shown to positively affect the lean body mass, the ADL and the quality of life (EQ-5D). The fracture healing complications had a negative impact on the body weight, the lean body mass and the quality of life [31].
A study was undertaken to evaluate the effect of an extract of Cissus Quadrangularis Linn (CQ) which contained high amounts of Vitamin C, carotene A, anabolic steroidal substances and calcium, on the healing process of experimentally fractured radiusulnas of dogs. The CQ treated animals revealed a faster initiation of the healing process than the control animals on radiological and histopathological examinations. The treated group also revealed a decrease in the serum calcium levels to a greater extent than the control group. The healing was almost complete on the 21st day of the fracture in the treated animals and it remained incomplete in the control animals [32].
Anabolic steroids, especially oxandrolone, have been successfully used in the trauma and the burn patient population to decrease the lean mass loss in the acute phase of the injury, as well as, to more rapidly restore the lost lean mass in the recovery phase. Several studies have demonstrated an increase in the healing of chronic wounds. However, significant lean mass gains were also present [33]. Falanga et al., [34] reported a stimulation of the collagen synthesis with the anabolic steroid, stanazol. Erlich et al., [35] reported a 10-fold increase in the messenger RNA for collagen synthesis in a human fibroblast culture with oxandrolone. Tenenbaum et al., [36] reported an increased synthesis of bone, collagen, matrix, and epidermis in a wound of the oral cavity which was stimulated with oxandrolone. A histological analysis in our study also revealed more densely packed collagen with higher number of fibroblasts and mononuclear cells. Anabolic steroids have also been shown to trigger the release of the transforming growth factor beta by fibroblasts, which stimulates the bone formation in the granulation tissues [37].
CONCLUSION
From the above facts, we can conclude that anabolic steroids produce a better fracture healing by exaggerating the periosteal bone formation and by achieving a high osteoblastic activity and a better mineralization of the callus. They also seem to exert mild inhibition of the bone resorption without affecting or even stimulating the bone formation. Thus, anabolic steroids will definitely improve the fracture healing outcome and reduce the morbidity and the negative socio-economic impact. This was an initial study which was done to test the effect of Nandrolone decanoate on fracture healing, which showed positive results. We intend to do future evaluation with multiple doses and for a longer duration of time.
[1]. Ferguson C, Alpern E, Miclau T, Helms JA, Does adult fracture repair recapitulate embryonic skeletal formation?Mech Dev. 1999 87:57-66. [Google Scholar]
[2]. Einhorn TA, The cell and molecular biology of fracture healingClin Orthop Relat Res. 1998 S7-S21. [Google Scholar]
[3]. Gerstenfeld LC, Cho TJ, Kon T, Aizawa T, Tsay A, Fitch J, Impaired fracture healing in the absence of TNF-alpha signaling: the role of TNF-alpha in endochondral cartilage resorptionJ Bone Miner Res. 2003 18:1584-92. [Google Scholar]
[4]. Einhorn TA, The science of fracture healingJ Orthop Trauma. 2005 19:S4-6. [Google Scholar]
[5]. Hedstrom M, Saaf M, Dalen N, Low IGF-I levels in hip fracture patients: a comparison of 20 coxarthrotic and 23 hip fracture patientsActa Orthop Scand. 1999 70:145-48. [Google Scholar]
[6]. Patterson B, Cornell C, Carbone B, Levine B, Chapman D, Protein depletion and metabolic stress in elderly patients who have a fracture of the hipJ Bone Joint Surg Am. 1992 74-A:251-60. [Google Scholar]
[7]. Hillier S, Cooper C, Kellingray S, Russell, G, Hughes, H, Coggon, D, Fluoride in drinking water and risk of hip fracture in the UK: a case control studyLancet 2000 22:265-69. [Google Scholar]
[8]. Thibaud D, Burckhardt P, Costanza M, Importance of albumin, 25(OH)-vitamin D and IGFBP-3 as risk factors in elderly women and men with hip fractureOsteoporosis Int. 1997 7:457-62. [Google Scholar]
[9]. Delmi M, Rapin CH, Bengoa JM, Delmas PD, Vasey H, Bonjour JP, Dietary supplementation in elderly patients with fractured neck of the femurLancet 1990 335:1013-16. [Google Scholar]
[10]. Sernbo I, Johnell O, Consequences of a hip fracture: a prospective study over 1 yearOsteoporos Int. 1993 3:148-53. [Google Scholar]
[11]. Hedström M, Gröndal L, Örtquist Å, Dalén N, Ahl T, Serum albumin and deep infection in femoral neck fractures: a study of 437 cases followed for one yearInt Orthop. 1998 22:182-84. [Google Scholar]
[12]. Fox KM, Magaziner J, Hawkes WG, Yu-Yahiro, J, Hebel, JR, Loss of bone density and lean body mass after hip fractureOsteoporosis Int. 2000 11:31-35. [Google Scholar]
[13]. Kay SP, Moreland JR, Schmitter E, Nutritional status and wound healing in lower extremity amputationClin Orthop. 1987 217:253-56. [Google Scholar]
[14]. Puskarich CL, Nelson CL, Nusbickel FR, Stroope HF, The use of two nutritional indicators in identifying long bone fracture patients who do and do not develop infectionsJ Orthop Res. 1990 8:799-803. [Google Scholar]
[15]. Berger JR, Pall L, Hall CD, Simpson DM, Berry PS, Dudley R, Oxandrolone in AIDS-wasting myopathyAIDS 1996 10:1657-62. [Google Scholar]
[16]. Ferreira IM, Verreschi IT, Nery LE, Goldstein RS, Zamel N, Brooks D, The influence of 6 months of oral anabolic steroids on body mass and respiratory muscles in undernourished COPD patientsChest 1998 114:19-28. [Google Scholar]
[17]. Monfoulet L, Rabier B, Chassande O, Fricain JC, Drilled hole defects in mouse femur as models of intramembranous cortical and cancellous bone regenerationCalcif Tissue Int. 2010 86:72-81. [Google Scholar]
[18]. Campbell TM, Wong WT, Mackie EJ, Establishment of a model of cortical bone repair in miceCalcif Tissue Int. 2003 73:49-55. [Google Scholar]
[19]. Corrales LA, Morshed S, Bhandari M, Miclau III T, Variability in the assessment of fracture-healing in orthopaedic trauma studiesJ Bone Joint Surg Am. 2008 90:1862-68. [Google Scholar]
[20]. Hiltunen A, Vuorio E, Aro HT, A standardized experimental fracture in the mouse tibiaJ orthop Res. 1993 11(2):305-12. [Google Scholar]
[21]. Yi-Xin He, Ge Zhang, Xiao-Hua Pan, Zhong Liu, Li-zhen Zheng, Chun-Wai Chan, Impaired bone healing pattern in mice with ovariectomy-induced osteoporosis: A drill-hole defect modelBone 2011 48(6):1388-1400. [Google Scholar]
[22]. Aerssens J, Van Audekercke R, Geusens P, Schot LPC, Osman AA, Dequeker J, Mechanical properties, bone mineral content, and bone composition (collagen, osteocalcin, IGF-I) of the rat femur: influence of ovariectomy and nandrolon decanoate (anabolic steroid) treatmentCalcif Tissue Int. 1993 53(4):269-77. [Google Scholar]
[23]. Carrazzo M, Evaluation of the effect of anabolic steroids, calcitonin and 25-hydroxycholecalciferol on the spongy bone of ratsRev Rhum Mal Osteoarthritis 1985 32(1):17-19. [Google Scholar]
[24]. Erdtsieck RJ, Pols HA, van Kuijk C, Birkenhäger-Frenkel DH, Zeelenberg J, Kooy PP, Course of bone mass during and after hormonal replacement therapy with and without addition of nandrolone decanoateJ Bone Miner Res. 1994 9(2):277-83. [Google Scholar]
[25]. Hassanger C Christiansen, Epidemiology, biochemistry and some results with treatment of postmenopausal osteoporosisWien Med Wochenshr 1993 143(14-15):389-99. [Google Scholar]
[26]. Johansen JS, Treatment of postmenopausal osteoporosis: is the anabolic-steroid nandrolone decanoate a candidate?Bone Miner. 1989 6(1):77-86. [Google Scholar]
[27]. Granstrom G, Nilsson LP, Experimental mandibular fracture: Studies on bone repair and remodellationScand J. Plast Reconstr Surj Hand Surg. 1987 21(2):159-65. [Google Scholar]
[28]. Frankle M, Borrelli J, The effects of testosterone proprionate and methenolone enanthate on the healing of humeral osteomies in the Wistar RatJ. Invest. Surg. 1990 3(2):93-113. [Google Scholar]
[29]. Lyritis G, Papadopoulou Z, Nikiforidis P, Batrinos M, Varonos D, Effect of cortisone and an anabolic steroid upon plasma hydroxyproline during fracture healing in rabbitsActa Orthop Scand. 1975 Apr 46(1):25-30. [Google Scholar]
[30]. Viljanto J., Isomäki H, Kulonen E, Effect of an anabolic steroid on the tensile strength of granulation tissue in various nutritional statesActa Endocrinol. November. 1, 1962 41:395-99. [Google Scholar]
[31]. Tidermark J, Quality of life and femoral neck fracturesActa Orthop Scand Suppl. 2003 Apr 74(309):1-42. [Google Scholar]
[32]. Deka DK, Lahon LC, Saikia J, Mukit A, Effect of clssus quadrangular/s in accelerating healing process of experimentally fractured radius-ulna of dog: a preliminary studyIndian journal of pharmacology 1994 26:44-45. [Google Scholar]
[33]. Demling Robert H, The Role of Anabolic Hormones for Wound Healing in Catabolic StatesJournal of Burns and wounds4:46-62. [Google Scholar]
[34]. Falanga V, Greenberg A, Zhou L, Stimulation of collagen synthesis by the anabolic steroid stanazolJ Invest Dermatol 1998 11:1193-97. [Google Scholar]
[35]. Erlich P, The influence of the anabolic steroid oxandrolone upon the expression of procollagen types I and II MRNA in human fibroblasts cultured in collagen or plasticWounds 2001 13:66-72. [Google Scholar]
[36]. Tennenbaum R, Shkear G, Effect of the anabolic steroid oxandrolone on wound healingOral Surg. 1970 30:834-35. [Google Scholar]
[37]. Demling RH, Oxandrolone, an anabolic steroid, enhances the healing of a cutaneous wound in the ratWound Repair Regen. 2000 8:97-102. [Google Scholar]