The incidence of nosocomial infections in the intensive care units (ICU) is showing a rising trend, mainly because of the severe clinical conditions which are associated with the impaired immunity, increasing the use of invasive diagnostic procedures, lapses in the sterilization and the disinfection techniques and the indiscriminate use of antibiotics. The β-lactam antibiotics are among the most frequently prescribed antibiotics in the ICUs world-wide, which are favoured because of their efficacy, broad spectra and low toxicity. The selective pressures which are generated by the indiscriminate use of the beta-lactam antibiotics have led to the selection of a variety of mutated forms of β-lactamases such as the ESBLs, AmpC β-lactamases and metallo-β-lactamases which have emerged as the most worrisome resistance mechanism which poses a therapeutic challenge to the health care settings [1]. These “newer β-lactamases” are capable of hydrolyzing a wide range of β-lactam antibiotics, notably the extended-spectrum penicillins and the third and fourth generation cephalosporins, which include the carbapenams [2]. The ESBL producing organisms also express the AmpC β-lactamases and they may be co-transferred with the plasmids, thus mediating the fluoroquinolone and the aminoglycoside resistance. The treatment options are fast running out, particularly against the gram negative nosocomial pathogens [3]. They are of significant concern because they restrict the therapeutic options, cause treatment failures and are increasing in occurrence worldwide. These enzymes are associated with the potentially fatal laboratory reports of a false susceptibility to the cephalosporins, that can lead to the prescription of the inappropriate therapy for the infected patients [4]. The detection of the metallo beta lactamase and the Amp C mediated resistance in the clinical microbiology laboratory poses a problem, because the phenotypic tests are not standardized. The Clinical Laboratory Standards Institute (CLSI) has not yet published the guidelines for their detection.
Though a number of phenotypic methods have been proposed, the coexistence of different classes of β-lactamases in a single bacterial isolate may pose diagnostic and treatment challenges. Hence, it is necessary to know the accurate prevalence of the β-lactamase producing strains in the high risk areas, so as to formulae a policy of the empirical therapy in the ICUs where the infections which are caused by the resistant organisms are much higher [5].
The present study was done to determine the prevalence rates of the multidrug resistant gram negative bacteria which produced the β-lactamase enzymes in various ICUs, so as to formulate an antimicrobial policy on the basis of the local epidemiological data.
MATERIAL AND METHODS
This study was conducted in the Department of Microbiology of the S.G.R.D Institute of Medical Sciences and Research, Amritsar, a rural tertiary care hospital of north India. A total of 273 gram negative, consecutive, non repetitive clinical isolates from 913 clinical samples, which were received from various ICUs over a period of one year were processed. All the isolates were identified by the standard microbiological tests. The antimicrobial susceptibility testing of the isolates was determined by the Kirby Bauer disc diffusion method according to the CLSI guidelines [6]. The reference strains, ESBL positive Klebsiella pneumonia ATCC 700603 and ESBL negative Escherichia coli ATCC 25922 were included in the study.
Detection of the ESBLs – Each strain was screened for the ESBL production against cefotaxime, ceftazidime and cefpodoxime. The strains which were resistant to these third generation cephalosporins were confirmed by three phenotypic tests i.e. the disc potentiation test (by using ceftazidime and ceftazidime-clav discs), the double disc synergy test and MIC reduction as per the CLSI guidelines [6].
Detection of the ampc β-lactamases – All the strains were screened for the AmpC β-lactamase production by the disc antagonism test. The isolates which showed a reduced susceptibility to cefoxitin were tested for confirmation by the modified three dimensional test. An indentation or a flattening of the zone of inhibition indicated the AmpC production [7].
Detection of the Metallo- β- lactamases (MbLs) – The metallo- β- lactamase production was detected by the ceftazidime – EDTA and the imipenam – EDTA double disc synergy test. The organisms were considered to be MBL producers if the increase in the inhibition zone of the beta lactam+EDTA disk was ≥ 5 mm [8].
RESULTS
The urinary tract infections (45.9%) were the most common infections, followed by skin and soft tissue infections (24.04%), respiratory tract infections (17.4%) and blood stream infections (12.02%) in the ICUs. Among the 273 gram negative isolates, the β lactamase production was detected in 193 (70.69%) isolates and the prevalence of the beta lactamases in the respective ICUs was determined. It was found to be maximum in the paediatric ICU (66.7%), the burns ICU (64.8%), the medical ICU (61.9 %), the surgical ICU (60%) and the neonatal ICU (45.4%).
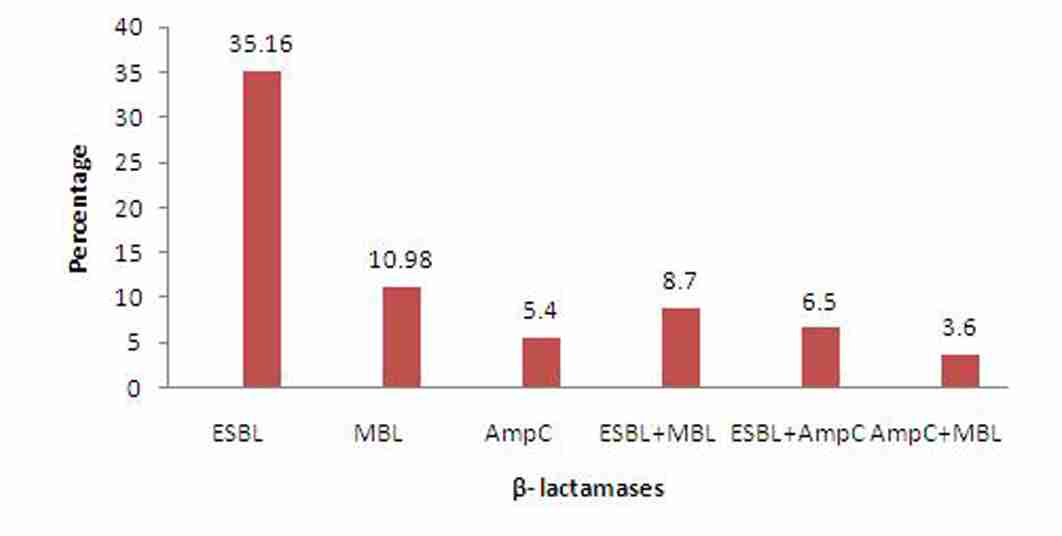
Of the 273 gram negative isolates, 96(35.16%) were ESBL producers, followed by 30(10.98%) metallo β-lactamase (MBL) producers and 15(5.4%) AmpC producers. The major ESBL producer was Escherichia coli (56.25%), followed by Pseudomonas aeruginosa (18.75%) and Klebsiella pneumoniae (15.62%).
The AmpC production was also maximally seen in Escherichia coli (86.67%), while the MBL production was mainly observed in Klebsiella pneumoniae (33.4%) and Pseudomonas aeruginosa (26.67%).
The co production of the ESBL/MBL/ AmpC β- lactamases was observed in 52 (19.04%) strains. The ESBL and MBL co production was detected in 24 (8.79%) isolates and it was found to be maximum in Escherichia coli (33.34%), Pseudomonas aeruginosa (25%) and Klebsiella pneumoniae (16.67%), while the ESBL and the AmpC co producers were 18 (6.59%) and they were commonly isolated from Escherichia coli (50%), Klebsiella pneumoniae (22.23%) and Acinetobacter baumannii (16.67%). The co production of AmpC and MBL was observed in 10 (3.67%) strains and it was detected mostly in Escherichia coli (60 %) [Table/Fig-1], [Table/Fig-2].
Distribution of various β-lactamases in clinical isolates [E/M—ESBL & MBL, A/M—AmpC & MBL, E/A—ESBL & AmpC co producers]
| Organism | Total | ESBL | MBL | Amp C | E/M | A/M | E/A |
| Isolates | No. | % | No. | % | No. | % | No. | % | No. | % | No. | % |
| E coli | 134 | 54 | 56.25 | 7 | 23.34 | 13 | 86.67 | 8 | 33.34 | 6 | 60.0 | 9 | 50.0 |
| Pseudomonas | 50 | 18 | 18.75 | 8 | 26.67 | - | - | 6 | 25.0 | 1 | 10.0 | - | - |
| Klebsiella | 44 | 15 | 15.62 | 10 | 33.34 | 1 | 6.67 | 4 | 16.67 | 1 | 10.0 | 4 | 22.2 |
| Citrobacter | 24 | 6 | 6.25 | 4 | 13.34 | 1 | 6.67 | 4 | 16.67 | 1 | 10.0 | 2 | 11.1 |
| Acinetobacter | 15 | 2 | 2.0 | 1 | 3.34 | - | - | 2 | 8.34 | 1 | 10.0 | 3 | 16.6 |
| Enterobacter | 4 | - | - | - | - | - | - | - | - | - | - | - | - |
| Proteus | 2 | 1 | 1.04 | - | - | - | - | - | - | - | - | - | - |
| Total | | 96 (35.16) | | 30 (10.98) | | 15 (5.49) | | 24 (8.79) | | 10 (3.67) | | 18 (6.59) | |
Prevalence of β- lactamase producing isolates in ICUs
| Organism | Total no. of GNB | Resistant Strains | β-lactamase producers (%age) |
| E coli | 134 | 97 | 73.38 |
| Pseudomonas | 50 | 33 | 66.00 |
| Klebsiella | 44 | 35 | 79.54 |
| Citrobacter | 24 | 18 | 75.00 |
| Acinetobacter | 15 | 9 | 60.00 |
| Enterobacter | 4 | - | - |
| Proteus | 2 | 1 | 50.00 |
| Total | 273 | 193 | 70.69 |
Of the 44 strains of Klebsiella pneumoniae, 35 were β lactamases producers (79.54%). The other major β lactamases producers were Citrobacter freundi (75.0%) and Escherichia coli (73.38%). [Table/Fig-3].
Distribution of β- lactamases in ICUs
A high degree of co-resistance to ciprofloxacin (79.6%), levofloxacin (62.7%), chloramphenicol (69.4%) gentamicin (74.3%) and amikacin (58.2%) was observed in the β lactamase producing organisms.
All the ESBL/Amp-c/MBL positive isolates were moderately sensitive to imipenam (59.7%) and the piperacillin + tazobactem combination (52.6%).
DISCUSSION
The infections which are caused by multidrug-resistant gram negative bacilli that produce various β lactamase enzymes have been reported with an increasing frequency in the intensive-care units and they are associated with a significant morbidity and mortality [9]. The numerous β- lactamases are encoded either by the chromosomal genes or by the transferable genes which are located on the plasmids or the transposones [10]. Initially, these enzymes were commonly found in the Klebsiella species and in E,coli [11] but now, these enzymes are produced by all members of Enterobacteriaceae and other gram negative bacilli [12].
In our study, the prevalence of various β lactamases in the gram negative bacteria, which included the Enterobactericeae and the nonfermenters was 70.69%, which was alarmingly high. The ESBL production was (35.16%) found to be maximum as compared to the other β lactamases. Similar findings were reported in a study which was done by Bandekar et al, which showed a high prevalence of the ESBL producers (39.8%) in burn patients [13].
A study which was done by Harakuni et al reported a high prevalence of the ESBLs (74%) in ICU patients [14]. Laghawe et al, in his study, reported 19.67% ESBL producers [15]. It has been proved that the prevalence of the ESBLs among the clinical isolates varies from country to country and institution to institution within the same country. In our study, the AmpC production was seen in 5.4% isolates as compared to that in other studies that had reported a high prevalence of the AmpC producers. It was 17.3% in Kolkata [16] and 22.9% in a study which was done by Bandekar et al., [13] in burn patients, whereas a study which was done by Bhattacharjee et al showed 22% AmpC producing Pseudomonas aeruginosa [17].
The low prevalence of the AmpC producers in our study could be due to the differences in the geographical distribution, which may have produced variations in the prevalence of the β-lactamases which may have been present in the different organisms, which may have given rise to the varied resistance patterns. The only β-lactams which were active against the AmpC and the ESBL coproducers were the carbapenems; however, recently, the resistance to the carbapenems has been increasing, which is mostly due to the production of the metallo β-lactamases. In the index study, the MBL producers were 10.98%. Our findings were in concordance with the study which was done by Bandekar et al, who reported 15.7% MBL producers [13].
The coexistence of different classes of β-lactamases in a single bacterial isolate may pose diagnostic and treatment challenges. The AmpC producing organisms can act as a hidden reservoir for the ESBLs. Also, the high-level expression of the AmpC β-lactamases may mask the recognition of the ESBLs and it may result in a fatal and an inappropriate antimicrobial therapy.
The coexistence of ESBL and MBL was reported in 8.79% isolates, whereas the AmpC and the MBL co production was shown by 3.67% isolates and the AmpC and the ESBL co production was shown in 6.59% isolates. A study which was done by Arora et al reported the AmpC and MBL coproduction in 46.6% isolates and the ESBL and AmpC co production in 3.3% isolates [16].
The increase in the prevalence of the AmpC, MBL and the ESBL producing isolates may be indicative of the ominous trend of more and more isolates acquiring the resistance mechanisms, thus rendering the antimicrobial armamarium ineffective. In our study, the multidrug resistant strains showed coresistance to the fluoroquinolones and the aminoglycosides, but they were moderately susceptible to imipenam and the piperacillin- tazobactem combination, which was in concordance with the findings of other studies [18,19].
The high prevalence of these organisms in the ICUs emphasizes the need for an early detection of the β-lactamase producing organisms by simple screening methods, which can help in providing an appropriate antimicrobial therapy and in avoiding the development and the dissemination of these multidrug resistant strains. The need of the hour is that every health care institution must develop its own antimicrobial stewardship program which is based on the local epidemiological data and international guidelines, to optimize the antimicrobial use among the hospitalized patients, to improve the patient outcomes, to ensure a cost-effective therapy and to reduce the adverse consequences of the antimicrobial use [20]. Preventive measures like a continuous surveillance of the ICUs and a strict implementation of infection control practices can go a long way in containing the menace of drug resistance in the health care settings.