Peripheral Arterial Disease (PAD) is one of the most common and the important types of Peripheral Vascular Diseases (PVD) [1]. PAD is more prevalent in diabetics than in nondiabetics [2]. It is one of the many factors that contribute to the progressive and the critical courses of limb ischaemia in the Type 2 Diabetes Mellitus (DM) patients. The presence of lower extremity ischaemia in the type2DM patients is often suggested by a combination of the clinical signs and symptoms plus the abnormal results on several noninvasive vascular tests like the transcutaneous oxygen measurement, the ABI and the absolute toe systolic pressure. Among them, the ABI has a proven role, both in the diagnosis of PAD and in the baseline assessment of the individuals who are at a risk of cardiovascular diseases [1].
The ABI is a very simple, noninvasive test that can be performed easily in the office by using a handheld Doppler device. The Ankle-Brachial Index (ABI) is the ratio of the systolic pressure at the ankle to that in the arm. Generally, the higher systolic pressure in the dorsalis pedis and in the posterior tibial arteries serves as the numerator and the higher systolic pressure in the brachials serves as the denominator. A low ABI of 0.9 or < 0.9 is a useful diagnostic tool for detecting PVD and it is also considered as a strong predictor of the cardiovascular morbidity and mortality [3–6].
Furthermore, chronic inflammation is a new pathophysiologic determinant for Type2 DM, which is found to be associated with several inflammatory markers. The C-Reactive Protein (CRP) is the most reliable marker of the vascular inflammation [10,11]. CRP is a member of the pentraxin family of proteins which is characterized by a cyclic pentameric structure and a radial symmetry [12, 13]. CRP is a trace protein in the circulation of healthy subjects, with a median concentration of 1 mg/L. CRP is also a classical acute-phase protein which increases 1,000-fold in response to infection, ischaemia, trauma, burns, and inflammatory conditions and decreases just as rapidly with the resolution of the pathology. The acute phase phenomena may also accompany chronic inflammatory disorders [14]. There is a growing evidence that CRP plays an important role in the development and the progression of Type 2 DM [15].
However, the relationship between the levels of the circulating inflammatory markers and the risk of progressive atherosclerosis has been relatively undetermined. Therefore, the present work was undertaken to compare the ABI, a marker of the atherosclerotic burden and hsCRP, a marker of the inflammation in the Type 2 DM patients and in normal subjects and also to assess their association in the Type 2 DM patients and in the normal subjects.
MATERIALS AND METHODS
The present study consisted of 80 Subjects, 40 type 2DM cases and 40 age and sex matched normal subjects who were in the age group of 40-60 years. Based on the findings of the outcome variable of ABI, a retrospective power analysis was performed with a 95% statistical power, a 5% level of significance and a sample size of 40 in each group. A total sample size of 80 was sufficient for conducting a valid and a statistically feasible research. The type 2DM cases were recruited from those outpatients who attended the Department of Endocrinology at the M S Ramaiah Medical and Teaching Hospitals, Bangalore,India. The type 2 DM patients were diagnosed as per the WHO criteria [6] who had a > 5 yrs of the duration of diabetes. The subjects with a history of viral illness in the past one month, a history of a physical injury to one/both limbs in the past 15 days and amputation of any limb and those with foot ulcers, varicose veins and other types of PAD were excluded from the study. The details about DM, smoking, alcohol intake and the physical activity were collected by taking the history and by using a questionnaire. All the study subjects were screened for their general physical health to rule out any clinical disorders like hypertension, CAD and other causes of PAD that were likely to interfere with the study findings. An informed written consent was obtained from all the study subjects after all the potential risks and the procedures were explained to them. There was no financial liability on the study subjects for any of the investigations and the procedures which were performed. An ethical clearance was obtained from the institutional ethical committee for human research for conducting the study. The anthropometric measurements like the weight in kilograms, and height (to the nearest cm) were recorded in all the subjects. The Body Mass Index (BMI) was calculated by the formula, weight (kg)/height (m2).
THE ABI MEASUREMENT
The ABI values were measured by using the traditional Continuous Wave (CW) Doppler of NICOLET VERSALAB, USA, 2003. As per the manufacturer’s instructions, this instrument was of the auto scaling type and it did not require any calibration. The bi-directional flow sensitive probes were periodically checked against the required sensitivity standards and they were maintained as was described by the manufacturer. The probe cables were replaced periodically during the maintenance/following damage.
All the ABI measurements were performed with the subjects in the supine position, in a dimly lit temperature-controlled (23–250C) room. After the participants rested supine for 10 minutes, a blood pressure cuff was placed on their upper arms and it was inflated until no brachial pulse was detected by the Doppler device. The cuff was then slowly deflated until the Doppler-detected pulse returned (the systolic pressure). This maneouver was repeated on the leg, with the cuff being wrapped around the distal calf and the Doppler device being placed over the dorsalis pedis or the posterior tibial artery. The pressures were measured twice. The ABI tracing of one of the cases and the controls has been shown in [Table/Fig-1] and in [Table/Fig-2] respectively. The ABI was calculated by dividing the ankle systolic pressure by the brachial systolic pressure.
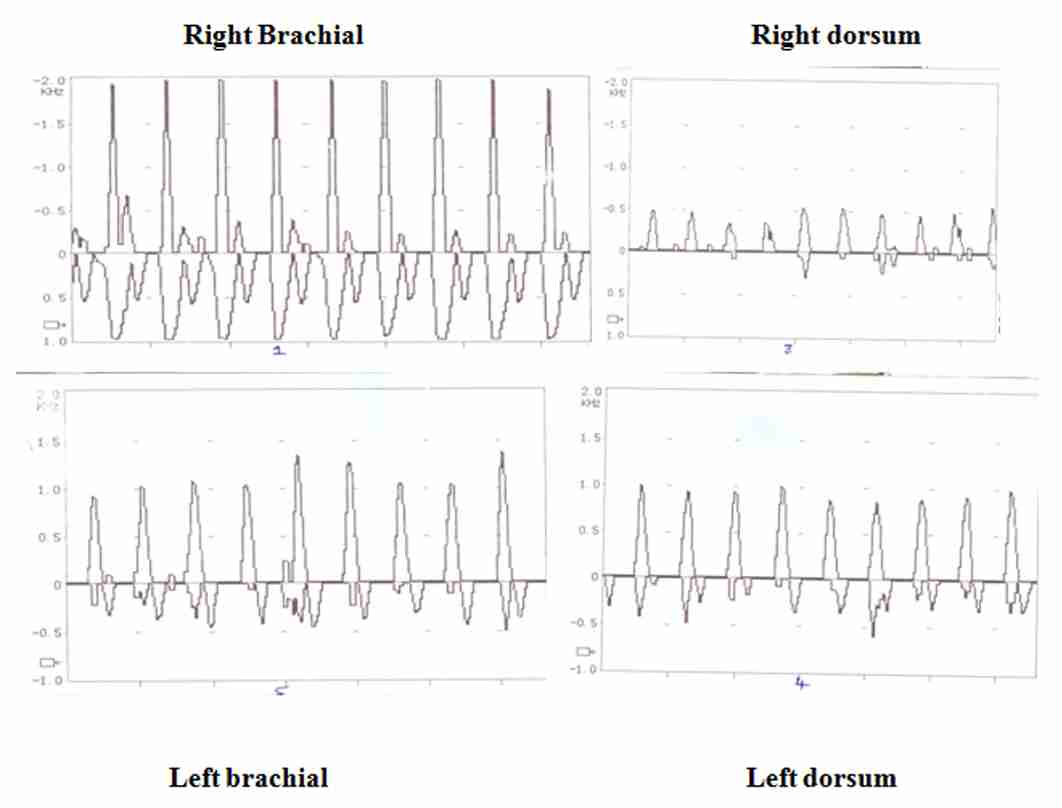
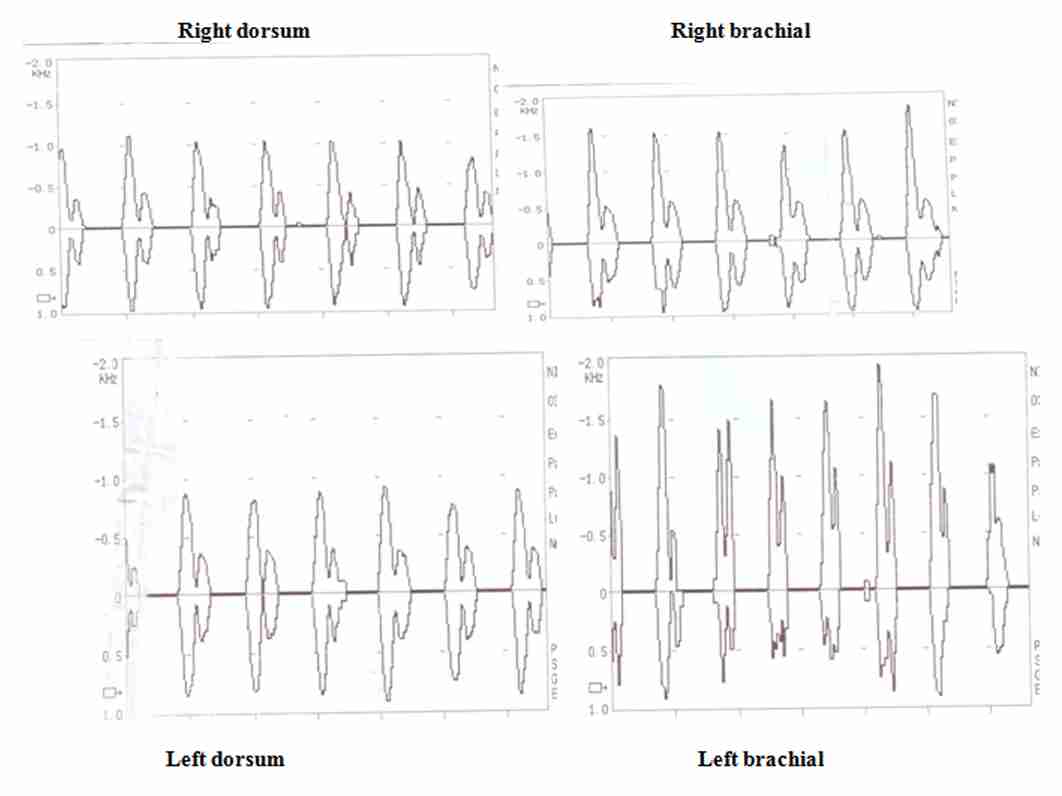
ASSAYS
5 ml of venous blood was collected from each study subject after a 12 hour overnight fast, by using vacutainers and disposable needles, from the antecubital vein, after taking the necessary aseptic precautions. The vacutainers which were used were commercially available, which contained a clot activator. The collected blood samples were left undisturbed for half an hour for complete clot formation. Then, the samples were centrifuged to separate the sera from the clots. After centrifugation, the sera were stored at -200C in Eppendorf tubes till the analysis was done. The fasting plasma glucose was determined by the glucose oxidase method by using a glucose autoanalyser. Glycosylated haemoglobin (HbA1c) was determined by the HPLC (High power liquid Chromatography) method and the serum hs-CRP levels were measured by the turbidimetry method.
STATISTICAL AnALYSIS: [
16,
17]
A descriptive statistical analysis was carried out in this study. The results on the continuous measurements were presented as mean ± SD and the results on the categorical measurements were presented in number (%). The significance was assessed at a 5 % level of significance. The Student’s t test (two tailed; independent) was used to find the significance of the study parameters between the normal and the Type 2 DM individuals. The Student’s t test (two tailed, independent) was used to compare the ABI in the Type 2 DM patients with that in the normal subjects. The Student’s t test (two tailed, independent) was used on a continuous scale and the Chi-square test/Fisher Exact test were used on the categorical dimensions to compare the hsCRP in the Type 2 DM patients with that in the normal subjects. Pearson’s Correlation was used to find the correlation of the serum hsCRP with the ABI in the Type 2 DM patients and in the normal subjects. The statistical softwares, namely SPSS, version 15.0, Stata 8.0, MedCalc 9.0.1 and Systat 11.0 were used for the analysis of the data and Microsoft Word and Excel were used to generate graphs, tables, etc.
RESULTS
This comparative study consisted of 40 Type 2 DM and 40 age and sex matched normal subjects who were in the age group of 40 – 60 years. The ABI and the serum hsCRP levels were measured and compared between the Type 2 DM and the normal subjects. Also, the association of the ABI and the highly sensitive C-Reactive Protein (hsCRP) was studied in the Type 2 DM and in the normal subjects.
The comparison of the basic characteristics like age, the male: female ratio, height, weight, and the BMI between the two groups did not show significant difference between the two groups (p > 0.05). The two groups were similar in terms of the basic characteristics [Table/Fig-3].
Comparison of study characteristics between two groups
| Study Characteristics (Mean ± SD) | Group a (controls) n=40 | Group B (cases) n=40 | Significance |
| Age in years | 53.89±4.23 | 55.58±4.06 | t=1.764; P=0.082 |
| Gender; Male:Female | 12:23 | 18:22 | x2=0.893;P=0.345 |
| Height (cm) | 158.26±7.95 | 157.30±15.45 | t=0.330; P=0.742 |
| Weight (kg) | 63.26±9.28 | 68.28±13.69 | t=1.830; P=0.071 |
| BMI (kg/m2) | 25.31±3.46 | 26.37±4.51 | t=1.124; P=0.264 |
The comparison of the study parameters like FPG, HbA1c and ABI showed a significant difference between the two groups (p < 0.05) whereas, hsCRP showed a near significant difference between the two groups (p = 0.069) [Table/Fig-4]. The ABI and hsCRP showed a significant negative correlation in the type 2 DM patients (r=- 0.560, p < 0.05) [Table/Fig-5] but such a correlation was not found in the normal subjects (r=0.253, p=0.143).
Comparison of Study parameters between two groups
| Study parameters | Controls | Cases | Significance |
| FPG (mg/dl) | 94.51±19.99 | 174.68±80.32 | t=5.745; P<0.001** |
| HbA1c | 5.45±0.89 (4.50-7.70) | 7.07±0.93 (4.60-9.50) | t=7.593; P<0.001** |
| ABI | 1.02±0.06 (0.95-1.18) | 0.97±0.12 (0.69-1.23) | t=2.144; P=0.035* |
| HsCRP mg/dl | 3.12±2.34 (0.01-7.06) | 4.51±3.85 (0.06-10.73) | t=1.848; P=0.069+ |
Pearson correlation between ABI and hsCRP in controls and cases
| ABI vs. HSCRP | Controls | Cases |
| r value | 0.253 | -0.560 |
| P value | 0.143 | <0.001** |
DISCUSSION
In this study, the ABI, a marker of the atherosclerotic burden and serum hsCRP, a marker of the inflammation were assessed and their association was studied in the patients with Type 2 DM and in the normal subjects. The two groups were similar in terms of the basic characteristics like age, sex and the BMI. The study parameters like HbA1c and FPG showed significant high values in the type2 diabetics as compared to those in the normals (P<0.001**). The important findings of this study were; the ABI was decreased and the hsCRP was raised in the type2 diabetics as compared to those in the normals. Also, there was a significant negative correlation between the ABI and hsCRP in the type 2DM patients as compared to those in the normal subjects.
The ABI is a simple, noninvasive test that objectively assesses the lower extremity arterial perfusion. It has a proven role, both in the diagnosis of PAD and in the baseline assessment of the individuals who are at a risk of cardiovascular diseases. A low ABI (<0.9), which is a useful diagnostic tool for detecting PAD, is also considered to be a strong predictor of the cardiovascular morbidity and the mortality in middle aged and older adults [6]. In short, a low ABI may reflect systemic atherosclerosis.
In the present study, the ABI was decreased in patients with Type 2DM as compared to that in the normals and it was statistically significant (P=0.035*). Since ABI is said to be a marker of the atherosclerotic burden, a low ABI in Type 2 DM suggested a significant atherosclerosis in the vascular bed. Furthermore: as a low ABI predicts about the cardiovascular morbidity and mortality, we can conclude that these patients were probably at a high risk of developing cardiovascular diseases in the future as compared to the type2 diabetics with a normal ABI and the non diabetic healthy controls. These findings were in agreement with those of Vicente I et al., [18] who concluded in their longitudinal study, that the prevalence of a low ABI was elevated in the type 2 diabetics and that this was related with the age, the duration of diabetes and the presence of vascular diseases.
In the present study, the serum hsCRP was also assessed by highly sensitive assays and it showed a trend towards a significant increase in the patients with type 2DM as compared to that in the normals (p=0.069+). This may be because of the small sample size in the present study. If a similar study is undertaken in a larger population, we may get a statistically significant increase in the values of hsCRP in the type 2DM patients. Secondly, as was observed in this study, even the normal subjects had slightly raised hsCRP levels (3.12±2.34) mg/dl, than the standard recommendations [19] which may have been due to the various contributing factors like genetic factors, age, BMI (25.31±3.46), etc. Since hsCRP is considered to be a highly sensitive marker of inflammation, a raised hsCRP level in the type 2 diabetics suggests that the inflammation may be involved in the pathogenesis of type 2DM. Furthermore, as hsCRP is now recognized as a powerful predictor of the cardiovascular events, we can also conclude that the type 2DM patients and the normals with raised hsCRP are probably at a high risk of developing cardiovascular diseases in the near future. These findings were in agreement with the results of earlier studies which were done by Francisco G et al., [15] who showed that the type 2DM patients had elevated serum hsCRP levels as compared to those in the normals.
Furthermore, in the present study, we also assessed the association of the ABI and hsCRP in both the groups, as the relationship between the inflammatory markers and atherosclerosis remains poorly defined. We found a significant negative correlation between the ABI and serum hsCRP in the type 2DM patients (r=- 0.560, p<0.001). However, such correlation was not found in the normal subjects (r=0.253, p=0.143). These findings were consistent with those of Santos S et al., [20] who also found a negative correlation between the inflammatory markers like the C-reactive protein, the white blood cell count,the lipoprotein-associated phospholipase A2 and the ABI.
The probable mechanisms which were involved in the association of high serum hsCRP with a low ABI in the type 2 diabetics may be because of the increased levels of the inflammatory markers, particularly CRP itself. This is because CRP is known to induce various inflammatory changes in the endothelial and the smooth muscle cells, that are associated with atherosclerosis [21]. Therefore, it is likely that an impaired endothelial function changes the mechanical properties of the vessel walls, leading to altered ABI values. Thus, CRP not only acts as a marker of inflammation but it is also involved in the initiation and the progression of atherosclerosis. Another possibility could be hyperglycaemia, as was reflected by the raised HbA1c levels in the type2 diabetics. The hyperglycaemia in type 2DM also induces inflammatory changes in the vascular endothelium, thereby leading to altered ABI values.
Thus, hyperglycaemia and inflammation jointly impair the endothelial function and probably lead to atherosclerosis, as was reflected by the low ABI, which in turn is indicative of PVD.
However, there have been conflicting findings regarding the relationship between hsCRP and the ABI. Yoshimasa Aso et al., [10] who studied the association of TBI and ABI with multiple blood markers of inflammation such as hsCRP, IL-6, and fibrinogen, found a significant negative correlation of TBI but not ABI with the serum hsCRP. They suggested that the falsely high ABI (>1.3) values which were due to a medial arterial calcification may account for the absence of a significant correlation between hsCRP and the ABI in the diabetic patients. But in the present study, we had no patient with high ABI values.
As the present study proves, an association between atherosclerosis, as indicated by a low ABI and inflammation, as was indicated by high serum hsCRP in the type 2 DM patients, It can be concluded that inflammation may be one of the processes which are involved in the pathogenesis of atherosclerosis, which in turn may lead to cardio vascular complications like Coronary Heart Disease (CHD), Myocardial Infarction (MI), ischaemic stroke, and Peripheral Vascular Disease (PVD) in the type2 diabetics and in the general population. Therefore, the assessment of the ABI and the serum hsCRP on a routine basis will help in identifying the subjects who are at a high risk of developing cardio vascular diseases. However, since this was a cross sectional study, we could not exactly conclude whether inflammation led to atherosclerosis or whether it just reflected the degree of the atherosclerotic vascular damage. Therefore, longitudinal studies need to be undertaken, to study the causal relationship between atherosclerosis and inflammation.