INTRODUCTION
Redox (reduction and oxidation) reactions are a family of reactions that are concerned with the transfer of electrons between molecules. Normally, the redox reactions ensure that the cells respond properly to endogenous and exogenous stimuli. During the cellular redox process, Reactive Species – Oxygen (ROS) and nitrogen (RNS)- are liberated as by-products. Lot of research has been directed to understand the beneficial and the deleterious effects of the reactive oxidizing molecules on the human body. An excess of these molecules is commonly referred to as ‘Oxidative stress’, which can lead to cell damage [1]. This may be one of the factors in the occurrence of many diseases like atherosclerosis, cataract, cardiovascular diseases, Alzheimer’s, etc [2–6]. Efforts are also on to design strategies to overcome oxidative stress by using antioxidants.
Reactive oxidizing molecules are the molecules with a strong oxidizing property. Free radicals, also called as radicals, are atoms which have unpaired electrons in their outer orbits. Because of this property, they are highly reactive. There are other molecules which lack free electrons in their outer orbits but are highly reactive. These molecules are called as non free radicals.
Because of their high reactivities, the free and the non free radicals are collectively called as Reactive Species.
Under the normal physiologic conditions, moderate levels of these reactive molecules allow their incorporation into the structure of the macromolecules in a reversible fashion. Such reversible oxidative modifications of lipids, proteins, or DNA play a crucial role in the physiologic processes such as the differentiation, maturation, and trafficking of the intracellular vesicles.
ROS and RNS are formed under normal physiological conditions as the products of the cellular metabolism [7]. ROS can be (i) generated during UV light irradiation and by X-rays and gamma rays (ii) produced during metal catalyzed reactions (iii) present in the atmosphere as pollutants (iv) produced by neutrophils and macrophages during inflammation, and (iv) the by-products of mitochondrial catalyzed electron transport reactions, and various other mechanisms [8].
Though many reactive molecules are formed in the body, the Reactive Oxygen Species (ROS) and the Reactive Nitrogen Species (RNS) are the Important ones [Table/Fig-1]. A delicate balance between these 2 antagonistic effects is very important for the proper functioning of cells. This balance is termed as redox homeostasis. The delicate balance between the ROS generation and elimination is maintained by many complex mechanisms.
Examples of reactive species [9].
| Free radicals | Particals, not free radicals |
| ROS (reactive oxygen species) | Superoxide, O2• - | Hydrogen Peroxide, H2O2 (Fenton’S Reaction) |
| Hydroxyl Radical, OH• | Hypochlorous Acid, Hclo |
| Peroxyl, ROO • | Ozone, O3 |
| Alkoxyl, RO • | Singlet Oxygen, 1O2 |
| Hydroperoxyl, HO2• | |
| RNS (Reactive nitrogen species) | Nitrogen(II) Oxide, NO. | Nitrosyl, NO+ |
| Nitrogen(IV) Oxide, NO2. | Nitrous Acid, HONO |
| Nitogen(III) Oxide, N2O3 |
| Peroxynitrite, ONOO – |
| Alkylperoxinitrite, ROONO |
The reactive oxygen species can be beneficial, as they participate in various redox-regulatory mechanisms of the cells in order to protect the cells against oxidative stress and the maintenance of the cellular “redox homeostasis” [Table/Fig-1]. They also act as second messengers, controlling various normal physiological functions of the organism [10].
The free radicals become deleterious when they are not eliminated by the endogenous systems. Oxidative stress represents an imbalance between the production of reactive species and a biological system’s ability to readily detoxify the reactive intermediates or to repair the resulting damage. Detoxification of the reactive radicals occurs through antioxidants.
An excess of the reactive molecules cause severe and irreversible oxidative damages. This mini-review deals with the production of the reactive species and their physiological roles; type and location and the role of anti-oxidants along with the role of the reactive species and anti-oxidants in cancer.
Oxidative stress can be caused due to the depletion/reduced activity of the antioxidants or an increase in the reactive species [11].
ANTIOXIDANTS
The antioxidant compounds are exogenous or endogenous in nature. They prevent the generation of/ intercept and inactivate the already formed oxidants. Thus they block the chain of the reactions which is produced by these oxidants. An antioxidant is any substance, when present at low concentrations, significantly delays or inhibits an oxidation reaction’. The antioxidants are of two types, non-enzymatic and enzymatic [12].
TYPES
1) The enzymatic antioxidant defences include
Superoxide Dismutase (SOD), catalase (CAT), Glutathione Peroxidase (GPx) and glutathione reductase.
2) The non-enzymatic antioxidants are
a) Metabolic antioxidants- glutathione (GSH), Lipoid acid, L-arginine, coenzyme Q10, melatonin, uric acid, bilirubin, metal chelating proteins, transferrin, etc.
b) Nutrient antioxidants- ascorbic acid (Vitamin C), tocopherol (Vitamin E), carotenoids, trace metals (selenium, manganese,zinc) flavonoids,omega-6 fatty acids and other antioxidants. [Table/Fig-2] shows the various antioxidants with their locations and brief roles.
| Location and Roles of antioxidants [13]. |
| Antioxidants | Location/Sources | Role |
| Superoxide dismutase (SOD) | Cytosol, mitocondria, nucleus, plasma | Dismutation of superoxide to hydrogen peroxide (H2O2) |
| Catalase (CAT) | Peroxisomes | Dismutation of H2O2 to molecular oxygen and water |
| Glutathione peroxidase (GPx) | Cytosol, mitochondria | Reduction of H2O2 and other hydroperoxides, lipid peroxides, lipoxygenase products |
| Glutathione reductase (GR) | Cytosol, mitochondria | Reduction of low molecular weight disulfides |
| Glutathione (GSH) | A tripeptide is present in high concentrations in most eukyrotic cells. Present within the cytosol of cells and is the major intracellular non protein thiol compound | Substrate in GSH redox cycle, act as a reductant, reducing H2O2 directly to water with the formation of GSSG. It also reacts with superoxide anion,hydroxyl, and alkoxyl radical directly by a radical transfer process and inhibits tissue damage. GSH is capable of scavenging ROS directly or enzymatically via GPx |
REDOX SIGNALLING AND REDOX HOMEOSTASIS
[Table/Fig-2,3 & 4] Redox signalling is the biochemical communication between the free radicals, Reactive Oxygen. Species (ROS), and other electronically activated species such as nitric oxide and other oxides of nitrogen, which act as biological messengers. Similarly, the modulation of the charge-transfer processes and the electronic conduction in the macromolecules is also redox signaling [14].
| Uric acid) | Wide distribution | Uric acid is a powerful antioxidant and is a scavenger of singlet oxygen and radicals like superoxide anion, hydroxyl, and alkoxyl radical and binds transition metals. |
| Cysteine | Wide distribution | Cysteine is also a vital component for the synthesis of glutathione and can reduce organic compounds by donating e- from SH groups. N-acetyl-L-cysteine (NAC) is a derivative of cysteine act as glutathione precursor and Scavenges of H2O2 and peroxide |
| CoQ 10 | Synthesized in human cells and also found in wheat bran, fish, meat. | It inhibit lipid peroxidation, reduces mitochondrial oxidative stress, and also able to recycle vitamin E. |
| Transferrin | It is a major iron transporting protein in the body | It bind free iron salts, which can leads to the generation of reactive oxygen species. |
| Lactoferin | It is a milk protein found extracellularlly | Similar action like transferring to helps in iron binding |
| Ceruloplasmin | A metal binging protein present extracellularlly | It is a copper binding protein. It catalyses the oxidation of Fe2+ to Fe3+ while oxygen is reduced to water |
| Bilirubin | Blood stream, tissue, plasma and extravascular place. It is Principal component of RBC. | It is an end product of heme catabolism, generally viewed as cytotoxic, lipidsoluble waste product. But at micromolar concentrations in vitro, efficiently scavenges peroxyl radicals and protects albumin-bound linoleic acid from peroxyl radical-induced oxidation. |
| Vitamin E | Present in high concentrations in both cells and mitochondrial membranes. Found in amla , lemon, oranges, groundnut, oil, olive oil, palm,oil, cashew nuts,germinated pulses. | Direct scavenging of superoxide,hydroxyl radicals, upregulation of antioxidant enzymes, breaks lipid peroxidation chain reactions. |
| Vitamin C | ICF, ECF, and also found in lemon,oranges, olive oil, palm oil, cashew nuts, germinated pulses. | Cysteine is also a vital component for the synthesis of glutathione and can reduce organic compounds by donating e- from SH groups. N-acetyl-Lcysteine (NAC) is a derivative of cysteine act as glutathione precursor and Scavenges of H2O2 and peroxide |
| Carotenoids: α-carotene, β-carotene, γ-carotene, crocin, β-cryptoxanthin, lycopene, lutein, zeaxanthin, bixin, astaxanthin, capsorubin, canthaxanthin | apples, banana, berries, grapes, jackfruit, kiwi, lemon, mango, pineapple, orange, papaya, plum, watermelon, sweet, beet,potato, carrot, asparagus, brinjal, broccoli, brussels tomatoes, sprouts, spinach, cauliflower, corn, onions, cabbage, beans, pumpkin, cucumber, mushroom, chillies, red palm oil, Milk, yogurt, eggs and medicinal plants. | Scavenges superoxide, hydroxyl radicals, neutralize oxidants from stimulated neutrophils, regenerates vitamin E. |
The redox state, like the pH or the osmotic pressure, represents the chemical characteristics of the intracellular environment. The free radicals and the reactive diamagnetic species which are derived from the radicals operate at low, but measurable concentrations in the cells. Each cell is characterized by a particular concentration of electrons (redox state) which is stored in many cellular constituents. The redox state of a cell and its oscillation determines the cellular functioning [15]. The term ‘redox signalling’ is used to describe a process in which the signal is delivered through redox reactions. Redox signalling requires that the steady state of the “redox balance” is disturbed, either by an increase in the ROS formation or by a decrease in the activity of the antioxidant system. The process of redox signalling is used for adapting the internal environment to the changes which occur extracellularly [7].
The essential cellular functions such as gene expression are influenced by the balance between the pro- and the antioxidant conditions. The number of reactive molecules is tightly controlled over a narrow range. The ability of the cell to maintain a condition of “steady state” or “redox homeostasis” is determined by the balance between the rate of its production and the rate of its removal by various antioxidants.
The intracellular “redox homeostasis” or “redox buffering” capacity is substantiated primarily by glutathione (GSH) and thioredoxin (TRX). Glutathione (2GSH/GSSG couple) represents the major cellular redox buffer and it therefore serves as an indicator of the redox environment of the cell [15,16].
The redox-mediated mechanisms in the regulation of the cellular processes
Transcriptional regulation
Direct oxidative modification
Regulation of the redox-sensitive interacting proteins
Regulation of the redox-sensitive modifying enzymes
Regulation of the protein turnover
The alterations in the redox homeostasis which are caused by exogenous stimuli or endogenous stress or both, can result in increased oxidative stress with elevated levels of cellular ROS. The ability to adapt to such ROS stress determines the overall fates of the cells [17].
Their exists a fine inter-relation between all antioxidants [Table/Fig-5].
Interrelationship between the antioxidant systems [19].
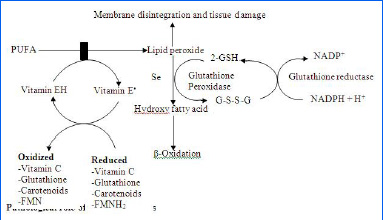
THE PHYSIOLOGICAL ROLE OF THE REACTIVE SPECIES
Numerous physiological functions are controlled by the redoxresponsive signalling pathways. The redox regulation involves the regulated production of Nitric Oxide (NO) or ROS by Nitric Oxide Synthases (NOS) or NAD(P)H oxidase respectively, and the effects of these compounds on the specific signalling cascades.
• NO• is generated in the biological tissues by specific NOSs which exist in 3 isoforms-neuronal (nNOS), endothelial (eNOS) which is constitutive and inducible (iNOS) which is not constitutive and can be induced. iNOS is expressed in the macrophages and if it is stimulated by lipopolysaccharides or cytokines, it can liberate NO as a defence mechanism. The cytotoxic effects of’ NO have been shown to be an important defence against parasitic fungi, protozoa, helminths and mycobacteria but not against the extracellular pathogens [18].
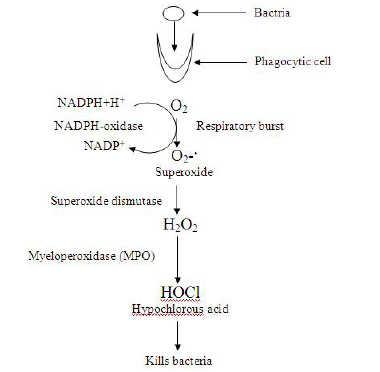
• Their Role (ROS) in Phagocytosis- Large amounts of super-oxide radicals and other ROS can be produced by the neutrophils and the macrophages during the process of phagocytosis [Table/Fig-6].
The respiratory burst during phagocytosis which is caused by neutrophils [19].
• Their role in the regulation of the cardiac and the vascular cell functioning- The non phagocytic cells like- fibroblasts, vascular smooth muscle cells, cardiac myocytes and endothelial cells can produce ROS intracellularly by NAD(P)H oxidase to regulate the intracellular signalling. ROS has been mainly shown to play a role in the regulation of the cardiac and the vascular cell functioning.
• Their role in the inhibition of platelet adhesion- H2O2 and NO• which are produced in the endothelial cells activate the enzyme, guanylate cyclase (sGC), which in turn, converts GTP to cGMP. cGMP modulates the function of the protein kinases and the ion channels and it regulates the smooth muscle tone and inhibits the platelet adhesion [20].
• Their role in the oxygen homeostasis- This is done by tight regulation of the RBC mass and the respiratory ventilation. The changes in the O2 concentration are sensed by several ROS producing proteins and they are regulated by some hormones like erythropoietin, the Vascular Endothelial Growth Factor (VEGF) and the insulin-like growth factor (IGF-II).
• Their role in cell adhesion- They play a role in the embryogenesis, wound repair, differentiation, etc. These processes are tightly redox regulated. The expression of the cell adhesion molecules are stimulated by various cytokines- Tissue Necrosis Factor (TNF), interleukin-1 (IL-1)and interleukin-1β.
• Their role in the activation of the immune response- Even small numbers of environmental pathogens can activate the immune response by involving the lymphocyte receptor for the antigens, the receptors for the co-stimulatory signals and various types of cytokines. The IL-2 production by the lymphocytes is increased by ROS [21,22].
• Their role in the programmed cell death (apoptosis)- Apoptosis is required for the proper development/destruction of the cells that represent a threat to the integrity of the organism. It depends upon the balance between the withdrawal of the positive signals- growth factors, interleukins, etc- and the receipt of negative signals- increased levels of oxidants, DNA damage, irradiation, etc. The ROS induced intracellular damage causes Bcl -2-a protein which is located in the outer membrane of the mitochondria, to set off a chain of reactions which leads to apoptosis, as shown in [Table/Fig-7].

• Their role in reproduction. During fertilization, the spermatozoa produce ROS for their maturation and membrane fusion with the oocyte. However, the excess generation of ROS by the spermatozoa or a reduced antioxidant defence have been implicated in male infertility [23].
• ROS in ageing17
Increasing evidence has suggested that ROS are involved in the process of biological ageing.
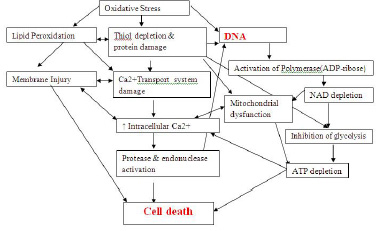
At low concentrations, the reactive species have a physiological role. At high concentrations, however, the reactive species may lead to permanent changes in the signal transduction and gene expression, which are typical for disease states. A high concentration of the reactive species is called as oxidative stress. At high concentrations, the reactive species mainly damage the proteins, nucleic acids and the lipids, as shown in [Table/Fig-8].
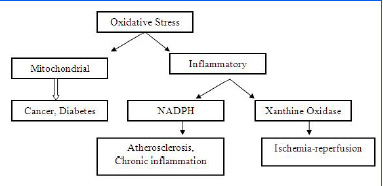
Oxidative stress induces a redox imbalance which may lead to an oncogenic stimulation [Table/Fig-9]. The ROS-mediated mutations and the loss of the critical regulatory mechanisms may lead to defective cell death and aberrant proliferation. These may contribute to the development of cancer [17]. The major events which contribute to carcinogenesis and the spread of cancer are-
Mechanism of Cell death which is caused by Oxidative stress [24].
Oxidative stress has been implicated in various pathological conditions [24].
DNA damage
Signal transduction abnormalities and
Remodelling of the extracellular matrix.
1. DNA damage- can occur because of- a protein damage (which includes the nuclear proteins) and lipid peroxidation Protein damage 7 - is generally the first step in carcinogenesis. Oxidative stress is responsible for the oxidation of the DNA proteins. This leads to mutations in the DNA, which are commonly seen in various tumours. The DNA damage involves single or double stranded DNA breaks and modifications in the DNA bases and/or DNA cross links. This leads to arrest or induction of the transcription; induction of the signal transduction pathways; replication errors and genomic instability- all of which can cause carcinogenesis.
In addition to ROS, RNS also can cause DNA damage. They mainly change guanine to 8-nitroguanine, which induces transversions of G: C to T: A in the DNA, which are unstable.
Mitochondrial DNA damage is also thought to be one of the factors in carcinogenesis. The ROS activate the transcription and the translation of the mitochondrial genome, which leaks out of the mitochondria and gets inserted in the nucleus, thus activating the oncogenes.
In addition to ROS, redox metals, due to their ability to generate free radicals or non-toxic metals and due to their ability to bind to critical thiols, are implicated in carcinogenesis. e.g.
Fe (Iron) – Colorectal Ca
Asbestos (30% Fe), Chromium – Lung Ca
Cadmium – Pancreatic/renal Ca
As (Arsenic) – acts by inhibiting the enzymes by binding to the SH groups and it is a well established co-carcinogen i.e. not causing cancer directly but increasing the ability of the other factors to cause DNA mutations, like- cigarette smoke, UV radiation, etc.
Lipid peroxidation- The various end products of lipid peroxidation like- malondialdehyde (MDA), 4-hydroxynonenal (HNE), acrolein, crotonaldehyde and 8-OH-G react with the DNA and produce mutagenic effects [25] [Table/Fig-10].
Effects of the end products of lipid peroxidation
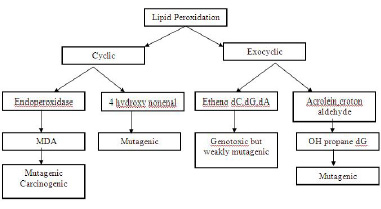
2. signal transduction abnormalities-
Signal transduction is the process by which the extracellular substances produce an intracellular response. The most fundamental process which is regulated by signal transduction is cell growth. So, deviations in the normal regulation of growth can lead to cancer. ROS interfere with the expression of a number of genes and the signal transduction pathways and they can thus cause cancer. The abnormalities of signal transduction are mainly because of the abnormal functioning of the receptors or of various nuclear transcription factors.
Abnormal functioning of the receptors: Several growth factor receptors are affected by ROS and carcinogenic metals like- nickel, arsenic, cobalt, etc. Activation of the extracellular growth factor is seen in lung and urinary carcinomas. Overexpression of the Platelet Derived Growth Factor (PDGF) is seen in lung and prostate cancers.
Nuclear transcription factors: The role of three factors is now well documented- AP-1, NF-kB and p53 [26]. AP-1- This factor is essentially responsible for the cell growth and differentiation. It also functions as a pro or an anti apoptotic agent, depending upon the balance between the pro and the anti-apoptotic genes which stimulate or inhibit AP-1. The duration of the stimulus is also important. In addition, AP-1 can interact with activated oncogenes and produce oncogenic transformation.
NF-kB- regulates several genes which are involved in cell transformation, proliferation and angiogenesis. During cell proliferation, NF-kB can exert dual effects- promotive as well as inhibitory. NF-kB is generally present in the cytoplasm in an inactive form because of its attachment to the inhibitory kB (IkB). ROS, Tumour Necrosis Factor (TNF) and interleukin-1 (IL-1) which are present during oxidative stress, stimulate NF-kB. On stimulation, it gets dissociated from the IkB, unmasks the nuclear localization sequence and enters the nucleus. It then binds to the kB regulatory elements and produces its effects [27].
p53- The tumour suppressor protein, p53, plays a key role in the development of malignancy. p53 exerts its effects by preventing the DNA damaged cells from dividing until a chromosomal repair has taken place. If the damage is not repaired, the cell undergoes apoptosis. A mutation in this gene which leads to its inactivation, is found in more than half of the tumours which are caused due to a direct action of the ROS or carcinogenic metals.
3. Remodelling of the extracellular matrix [7].
An important step in the growth of any tumour beyond a few millimetres, depends upon the development of new blood vessels. Angiogenesis and the development of metastasis are closely connected. Remodelling of the extracellular matrix is required for cancer cell invasion. Cancer cell invasion involves an interaction between the tumour cells and the surrounding stromal cells, leading to loss of the matrix function and the matrix boundary. Though many proteases may have a role in the remodelling, the activation of the zinc-dependent Matrix Metalloproteinases (MMPs) is the primary response. The MMPs degrade the components of the extracellular matrix. The MMPs are expressed only when a tissue remodelling occurs. Aberrant expressions of various MMPs have been correlated with tumour cell invasion and metastasis.
Angiogenesis involves an interaction between the endothelial cells, the surrounding pericytes and the smooth muscle cells and the ECM angiogenic cytokines. The MMPs degrade the basement membrane and other ECM components, thus allowing the endothelial cells to detach and migrate to new tissues. The MMPs also release pro-angiogenesis factors- the basic fibroblast growth factor (bFGF), VEGF and Transforming Growth Factor Beta (TGFβ). The MMP inhibitors have been shown to inhibit angiogenesis in various models.
The increased ROS stress can induce DNA mutations and genetic instability, which include a loss of the tumour-suppressor genes such as p53. The loss of the p53 function can in turn, further contribute to a mitochondrial dysfunction, ROS generation, and genomic instability, thus forming a vicious cycle. ROS stress may also induce the expression of the pro-survival factors and certain ROS scavenging proteins, which would enable the cells to adapt and survive under oxidative stress. The ROS-induced mutations and genetic instability further enhance the chance for the selection of the cells with malignant phenotypes (an increase in the proliferation, survival capacity, cell motility, and angiogenesis), leading to the development of cancer [28,29].
CONCLUSION
ROS, the antioxidants status and cancer- [30,31].
The antioxidants produce effects by scavenging the free radicals and by modulating the cell-signal pathways. Thus, antioxidants work in malignancy by many mechanisms such as -
Maintaining a normal cell cycle regulation
Inhibiting the cell proliferation and inducing apoptosis
Inhibiting the tumour invasion and angiogenesis
Reducing inflammation
Activating the detoxifying enzymes
Many studies have established the association of the disordered GSH-related enzyme functions and cancer. The Glutathione STransferases (GSTs) have been more frequently reported. The GSTs utilize glutathione in a wide range of reactions which involve carcinogens, drugs and products of oxidative stress [32]. As a measure of the redox homeostasis, the GSH/GSSG ratio is measured in many diseases. In colon and breast cancers, this ratio is significantly reduced, thus implying the presence of oxidative stress.
However, an antioxidant protection therapy should be used with caution in patients of malignancy. Free radicals are important in inducing apoptosis in cancers and the inhibition of this apoptosis might actually stimulate the survival of the damaged cells and their proliferation into a neoplastic state [33]. Increased glutathione levels in the late stages may give immunity to the cancer cells against chemotherapeutic agents. Another important issue is the pro oxidant character of some antioxidants, which may depend on the concentration and the environment. So, the usefulness of the antioxidant therapy needs proper evaluation before its initiation.
[1]. Yossi Gilgun-Sherki, Eldad Melamed, Daniel Offen, Oxidative stress induced-neurodegenerative diseases: the need for antioxidants that penetrate the blood brain barrierNeuropharmacology 2001 40:959-75. [Google Scholar]
[2]. Subash Vijaya Kumar, Saritha G., Fareedullah MD., Role of antioxidants and oxidative stress in cardiovascular diseasesAnnals of Biological Research 2010 1(3):158-73. [Google Scholar]
[3]. Agarwal A, Allamaneni Shyam SR, Oxidant and antioxidants in human fertilityMiddle East Fertility Society Journal 2004 9(3):187-97. [Google Scholar]
[4]. Bayani Uttara, Singh AV, Paolo Zamboni, Mahajan RT, Oxidative Stress and Neurodegenerative Diseases: A Review of Upstream and Downstream Antioxidant Therapeutic OptionsCurrent Neuropharmacology 2009 7:65-74. [Google Scholar]
[5]. Ilaria Guidia, Daniela Galimbertia, Silvia Lonatib, Cristina Novembrinoc, Fabrizia Bamontib, Marco Tiriticcoa, Oxidative imbalance in patients with mild cognitive impairment and Alzheimer’s diseaseNeurobiology of Aging 2006 27:262-69. [Google Scholar]
[6]. Maritim C, Sanders RA, Watkins JB, Diabetes, Oxidative Stress, and Antioxidants:A Review 2003 17(1):24-38. [Google Scholar]
[7]. Marian Valko, Dieter Leibfritz, Moncola Jan, Mark TD Cronin, Milan Mazura, Joshua Telser, Free radicals and antioxidants in normal physiological functions and human diseaseThe International Journal of Biochemistry & Cell Biology 2007 39:44-84. [Google Scholar]
[8]. Sen Saikat, Chakraborty Raja, Sridhar C., Reddy YSR, De Biplab, Free radicals, antioxidants, diseases and phytomedicines: current status and future prospectInternational Journal of Pharmaceutical Sciences Review and Research 2010 3(1):Article 021 [Google Scholar]
[9]. Halliwell Barry, Reactive Species and Antioxidants. Redox Biology Is a Fundamental Theme of Aerobic LifePlant Physiology 2006 141:312-22. [Google Scholar]
[10]. Foyer Christine H, Noctor Graham, Redox Homeostasis and Antioxidant Signaling: A Metabolic Interface between Stress Perception and Physiological ResponsesThe Plant Cell 2005 17:1866-75. [Google Scholar]
[11]. Chanda S., Dave R, In vitro models for antioxidant activity evaluation and some medicinal plants possessing antioxidant properties:An overview African Journal of Microbiology Research 2009 3(13):981-96. [Google Scholar]
[12]. Halliwell B, Gutteridge JMC, Free Radicals in Biology and Medicine 1989 2nd ednOxfordClarendon Press [Google Scholar]
[13]. Sen Saikat, Chakraborty Raja, The Role of Antioxidants in Human Health In Oxidative Stress: Diagnostics, Prevention, and Therapy; Andreescu, S., et al.;ACS Symposium Series 2011 Washington, DCAmerican Chemical Society [Google Scholar]
[14]. Emerit J, Samuel D, Pavio N, “Cu–Zn super oxide dismutase as a potential antifibrotic drug for hepatitis C related fibrosis”Biomedicine & Pharmacotherapy 2006 60(1):1-4. [Google Scholar]
[15]. Schafer FQ, Buettner GR, Redox environment of the cell as viewed through the redox state of the glutathione disulfide/ glutathione coupleFree Radic. Biol. Med. 2001 30:1191-212. [Google Scholar]
[16]. Droge, W, Free radicals in the physiological control of cell functionPhysiol. Rev. 2002 82:47-95. [Google Scholar]
[17]. Dunyaporn Trachootham, Weiqin Lu, Marcia A Ogasawara, Nilsa Rivera-Del Valle, Peng Huang, Redox Regulation Of Cell SurvivalAntioxidants and Redox Signaling 2008 10(8):1346-48.(new redox role) [Google Scholar]
[18]. Wink David A, Hines Harry B, Cheng Robert YS, Switzer Christopher H, Flores-Santana Wilmarie, Vitek Michael P, Ridnour Lisa A, Colton Carol A, Nitric oxide and redox mechanisms in the immune responseJ Leukoc Biol. 2011 89(6):873-91. [Google Scholar]
[19]. Naik Pankaja, In Biochemistry Chapter 29 Free radicals in Health Disease and Antioxidants3rd:595-600. [Google Scholar]
[20]. Datta K., Sinha S, Chattopadhyay P, Reactive oxygen species in health and diseaseThe National Medical Journal of India 2000 13:6 [Google Scholar]
[21]. Taner Yýlmaza, Elif Gülin Koçana, Beslerb H Tanju, The role of oxidants and antioxidants in chronic tonsillitis and adenoid hypertrophy in childrenInternational Journal of Pediatric Otorhinolaryngology 2004 68:1053-58. [Google Scholar]
[22]. Brambilla Daria, Mancuso Cesare, Scuderi Mariagrazia Rita, Bosco Paolo, Cantarella Giuseppina, Lempereur Laurence, A Review: The role of antioxidant supplement in immune system, neoplastic& neurodegenerative disorders: a point of view for an assessment of the risk/benefit profileNutrition Journal 2008 7:29 [Google Scholar]
[23]. Maneesh M, Jayalekshmi H, Role Of Reactive Oxygen Species And Antioxidants On Pathophysiology Of Male ReproductionIndian Journal of Clinical Biochemistry 2006 21(2):80-89. [Google Scholar]
[24]. Van Os C, Goris R, Bast A, Oxidative Stress and Cytotoxicity in Intestinal Epithelium. Printed by Print Partners Ipskamp, Enschede:1-127. [Google Scholar]
[25]. Halliwell Barry, Chirico Susanna, Lipid peroxidation: its mechanism, measurement, and significanceAm J Clin Nutr. 1993 57(suppl 7):15S-25S. [Google Scholar]
[26]. Johnson Renee F, Witzel Ini-Isabee, Perkins Neil D, p53-Dependent Regulation of Mitochondrial Energy Production by the RelA Subunit of NF-kBCancer Res71(16):5588-97. [Google Scholar]
[27]. Michael Karin, Yixue Cao, Florian R Greten, Zhi-Wei Li, NF-kB in cancer: from innocent bystander to major culpritNature Reviews Cancer 2002 2:301-10. [Google Scholar]
[28]. Wondrak Georg T, Comprehensive Invited Review on Redox-Directed Cancer Therapeutics: Molecular Mechanisms and OpportunitiesAntioxidants and Redox Signaling 2009 11(12) [Google Scholar]
[29]. Goodman Michael, Bostick Roberd M, Kucuk Omer, Jones Dean P, Review Article Clinical trials of antioxidants as cancer prevention agents: Past, present, and futureFree Radical Biology and Medicine 2011 51:1068-84. [Google Scholar]
[30]. Mauro Serafini, Debora Villano, Giovanni Spera, Nicoletta Pellegrini, Redox Molecules and Cancer Prevention: The Importance of Understanding the Role of the Antioxidant NetworkNutrition and Cancer56(2):232-40. [Google Scholar]
[31]. Foyera Christine H, Noctorb Graham, Redox sensing and signalling associated with reactive oxygen in chloroplasts, peroxisomes and mitochondriaPhysiologia Plantarum 2003 119:355-64. [Google Scholar]
[32]. Ortega Angel L, Mena Salvador, Estrela Jose M, Review Glutathione in Cancer Cell DeathCancers 2011 3:1285-310. [Google Scholar]
[33]. Halliwell B, Free radicals, antioxidants, and human disease: curiosity, cause or consequence?The Lancet 1994 344:721-24. [Google Scholar]