Insulin resistance, Menopause, Skin temperature measurements, HOMA index, QUICKI index
Introduction
Insulin Resistance (IR) is defined as a subnormal response to both endogenous and exogenous insulin [1]. It is characterized by a decreasing sensitivity of the target tissues to the action of insulin, by elevated blood glucose concentrations, and by the increased hepatic production of atherogenic lipids [2,3]. In addition to being associated with the termination of the reproductive life in women, menopause coincides with an increase in several co-morbidities, which include insulin resistance. The accumulation of the central abdominal fat in women at this time is associated with a decline in the production of a protein which is called adiponectin. Adiponectin, which is produced by fat, is important for the metabolism of glucose and fatty acids. In short, it makes the cells in the body, particularly the muscle and liver cells, more sensitive to the actions of insulin. Low serum adiponectin levels are associated with a condition which is called insulin resistance and the metabolic syndrome, such that the decline in adiponectin with the intra-abdominal weight gain at menopause is believed to play an important role in the development of insulin resistance after menopause [4]. In postmenopausal women, an increase in the insulin resistance is associated with an increased risk of diabetes, cardiovascular diseases and breast cancer [5]. There is a strong epidemiological evidence that the insulin resistance confers a significantly increased risk of cardiovascular disease which is independent of other cardiovascular risk factors [6]. Normally, insulin increases the Nitric Oxide (NO) synthesis at the site of the endothelium of the microcirculation and it causes vasodilatation in the skeletal muscle of healthy individuals [7]. NO is capable of attenuating a sympathetically induced cutaneous vasoconstriction [8]. The insulin resistance in postmenopausal women causes a decreased nitric oxide synthesis at the endothelium, favouring a sympathetically induced cutaneous vasoconstriction that leads to lowering of the skin temperature. Also, earlier studies which were based on the autonomic changes before and after oophorectomies in premenopausal women, have shown that oestrogen has a role in increasing the vagal action and in reducing the sympathetic action [9]. Therefore, the decreased values of the skin temperature measurements in postmenopausal women could indicate an increased sympathetic activity and a decreased insulin sensitivity. To reinforce the presence of the insulin insensitivity in the study group of postmenopausal women, the values of the fasting insulin and the fasting glucose levels were incorporated for the Homeostatic Model Assessment (HOMA) and the Quantitative Insulin Sensitivity Check Index (QUICKI). Although a HOMA score of 1.0 is the ideal one, the study of Bonora et al., [10] found a mean HOMA IR score of 2.06± 0.14 in the normal non diabetic population. In the absence of a local reference data for the HOMA IR score, we may assume a value of greater than 2.0 to represent the insulin resistance. For the Quantitative Insulin Sensitivity Check Index (QUICKI), a score of <0.35 (equivalent to HOMA >1.8) was used as an indicator of the IR.
Aim
To correlate the skin temperature measurements with the increased insulin resistance in post menopausal women and to confirm the results with the HOMA and the QUICKI indices.
Materials and Methods
This study was conducted in the Dept of Physiology, PSGIMS & R after getting a clearance from the institute’s ethics committee and after getting an informed consent from the study participants. 25 premenopausal and 25 postmenopausal women participated in this study.
Inclusion Criteria
Postmenarchal, premenopausal women of ages, 12-45 years, who were in the follicular phase of the menstrual cycle, who did not have any personal or family history of diabetes and hypertension.
Postmenarchal, premenopausal women without any menstrual irregularities.
Post menopausal women.
No women were to take confounding medications which were known to affect the sex steroids or the insulin action.
No women were to be on any medications which affected the lipid or the carbohydrate metabolisms.
Exclusion Criteria
Premenarchal women.
Control women who had any personal or family history of diabetes or hypertension.
Control women with menstrual irregularities.
Women who took confounding medications which were known to affect the sex steroids or the insulin action.
Women who were on any medications which affected the lipid or the carbohydrate metabolisms.
Analysis of the Skin Temperature
The skin temperature was assessed by using the Student Physiograph, with the help of a temperature coupler and a transducer. Initially, the transducer was allowed to stabilize to the room settings for about 20 min, by connecting it to the coupler. After its stabilization, the transducer was allowed to record the room temperature that was monitored by a thermometer. A graph was prepared with the lower baseline of the chart which was set as room temperature and the higher temperature baseline was obtained by immersing the transducer in boiling water. The calibration was done and the skin electrodes were placed on the subject to record the skin temperature, which was determined from the graph which was obtained.
Homeostatic model assessment (HOMA) [
11,
12]
HOMA has been widely employed in the clinical research to assess the insulin sensitivity. Rather than using fasting insulin or a G/I ratio, the products of the fasting values of glucose (expressed as mg/dL) and insulin (expressed as μU/mL) are divided by a constant:
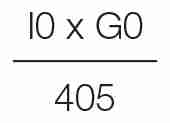
Quantitative Insulin Sensitivity Check Index (QUICKI) [
12,
13]
Like HOMA, QUICKI can be applied to the normoglycaemic and the hyperglycaemic patients. It is derived by calculating the inverse of the sum of the logarithmically expressed values of fasting glucose and insulin:
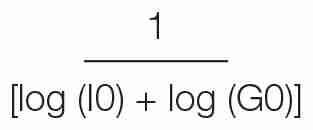
Statistical Analysis
The results were subjected to the appropriate statistic analyses and the significance was noted. This was a case control study and the association was found by applying Fischer’s exact test and the P value was estimated. The statistical significance was set at p < 0.05. The Student’s t test was applied to determine the significant differences in the skin temperature measurements.
Results
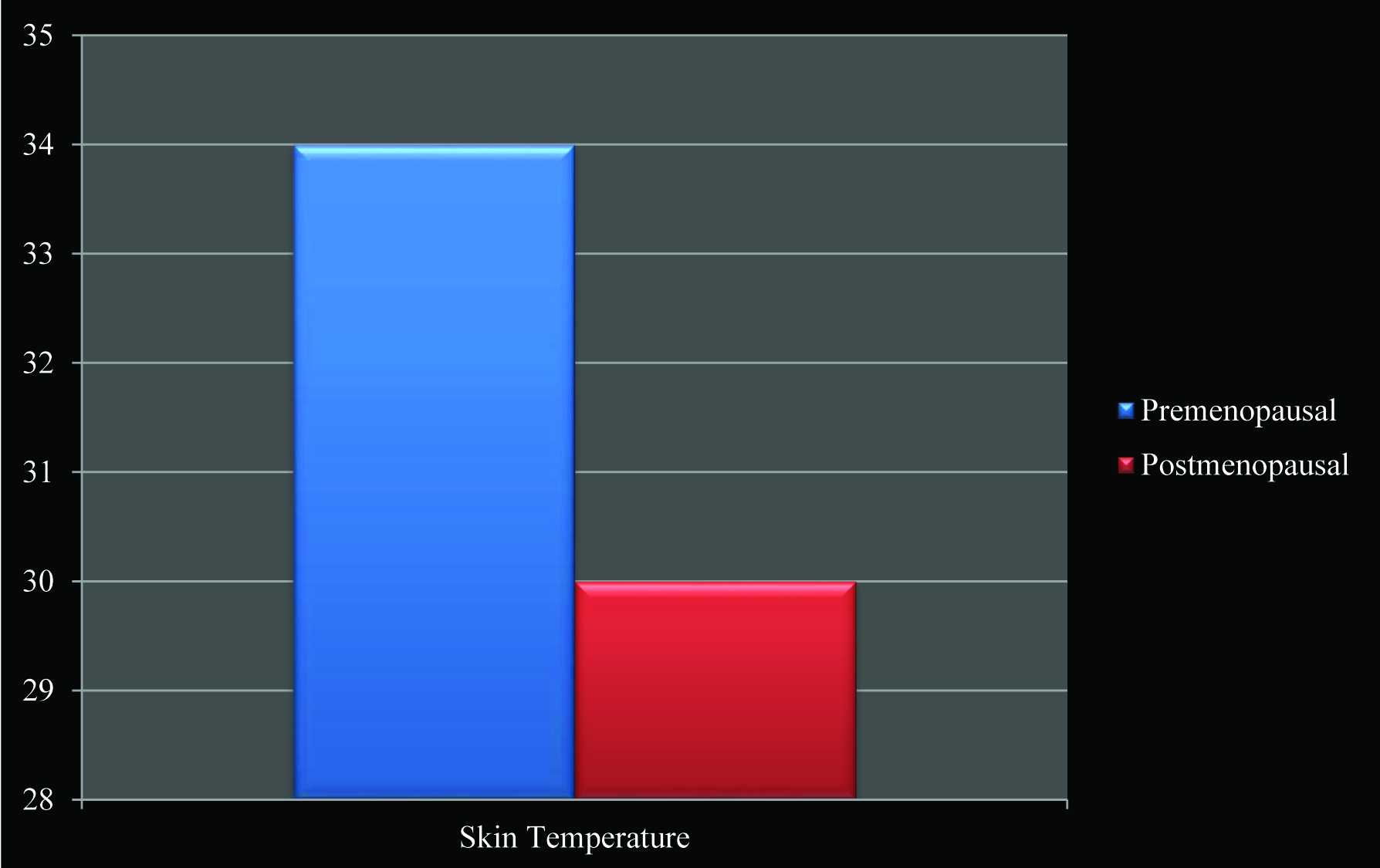
Both the HOMA index and the QUICK index were calculated and they were used to identify the likely IR individuals in the recruited population. By using the HOMA index and a cutoff point of > 2, only 15 subjects out of 25 post menopausal women were identified to be insulin resistant. Eleven of them were also identified by QUICKI, at a cutoff point < 0.357 [Table/Fig-1]. No premenopausal woman from the recruited population showed insulin resistance with the HOMA and the QUICKI indices [Table/Fig-2]. The skin temperature measurements showed a significant correlation with the HOMA and the QUICKI indices. There was a significant decrease (p value < 0.0001) in the skin temperature in the postmenopausal women as compared to that in the premenopausal women [Table/Fig-3]. The mean ± SD was found to be 0.3834 ± 0.1666 in the premenopausal women, and to be 2.192 ± 3.943 in the postmenopausal women.
Contingency table results for HOMA & QUICKI indices between premenopausal & postmenopausal women using Fischer’s exact test
| Parameter | P value |
|---|
| HOMA index | < 0.0001** |
| QIUCKI index | 0.0005* |
Highly Significant
Correlation coefficient between skin temperature measurements & HOMA and QUICKI indices
| Group | R value | P value |
|---|
| Skin temperature & HOMA | Premenopausal | -0.811 | 0.001** |
| Postmenopausal | -0.837 | 0.001** |
| Skin temperature & QUICKI | Premenopausal | 0.795 | 0.002** |
| Postmenopausal | 0.848 | 0.003* |
Highly Significant
Skin temperature measurements showed significant correlation with HOMA and QUICKI indices. Mean ± SD was found to be 0.3834 ± 0.1666 in premenopausal women, and 2.192 ± 3.943 in postmenopausal women.
There was a significant decrease (p value < 0.0001) in skin temperature in postmenopausal women when compared to premenopausal women
Discussion
The results which were obtained in this study showed a significant decrease in the skin temperature in the postmenopausal women as compared to that in the premenopausal women. This can be attributed to the increased insulin resistance in the postmenopausal women, which was suggested by the HOMA and the QUICKI indices. The low serum adiponectin levels are associated with a condition which is called insulin resistance and the metabolic syndrome, such that the decline in adiponectin with the intra-abdominal weight gain at menopause is believed to have an important role in the development of insulin resistance after menopause [4]. Menopause accentuates the storage of the excess abdominal fat and it may augment the development of the hepatic insulin resistance [14], whereas the diminished adiponectin levels which are linked to the increased adipose tissue [15] may be associated with the onset of both a hepatic and a peripheral insulin resistance [16]. Normally, insulin increases the nitric oxide synthesis at the site of the endothelium of the microcirculation and it causes vasodilation in the skeletal muscle of healthy individuals. But the insulin resistant subjects are characterized by endothelial dysfunction and endothelial resistance to insulin effect on enhancement of endothelium dependent vasodilatation [17]. NO alters the noradrenaline-induced cutaneous vasoconstriction at the postsynaptic level [18]. Moreover, the individuals who are relatively insensitive to the insulin-mediated glucose uptake also appear to have a corresponding decrease in the basal endothelial nitric oxide production [19]. Insulin resistance also produces a condition of hyperinsulinaemia. Hyperinsulinaemia has been associated with increased circulating levels of endothelin-1 [20]. Hyperinsulinaemia may affect the vascular functions through the activation of the systemic mechanisms [21] and by the increased endothelin activity [22]. These different effectors may ultimately impair the endothelium dependent dilation by reducing the vascular NO activity, possibly by increasing the oxidative stress [23]. Therefore, either a decrease in the NO production or a decreased activity of NO in the endothelium of the microcirculation causes a sympathetic vasoconstriction, which could explain the lowering of the skin temperature in the postmenopausal women with insulin resistance.
Conclusion
This study suggests a linear correlation between the skin temperature measurements and the insulin resistance. An increased prevalence of insulin resistance was seen in postmenopausal women as compared to that in premenopausal women. The insulin resistance was determined using the HOMA and the QUICKI indices. The increased insulin resistance correlated with the decreased skin temperature, which supported the primary aim of this study. There was a significant decrease in the skin temperature in postmenopausal women as compared to that in premenopausal women. Hence, the skin temperature could be considered as a parameter which suggests the insulin resistance.
**Highly Significant
**Highly SignificantSkin temperature measurements showed significant correlation with HOMA and QUICKI indices. Mean ± SD was found to be 0.3834 ± 0.1666 in premenopausal women, and 2.192 ± 3.943 in postmenopausal women.
Limitations of the study
One of the most important limitations of this study was the small sample size, since it was a short term ICMR project. A much larger group would have helped in a better interpretation of the results. A much larger longitudinal study is required to refine the results.
[1]. Fernandez-Real JM, Pickup JC., Innate immunity, insulin resistance and type 2 diabetesTrends Endocrinol Metab 2008 19:10-16. [Google Scholar]
[2]. Fonseca VA., Early identification and treatment of insulin resistance: impact on subsequent prediabetes and type 2 diabetesClin Cornerstone 2007 8Suppl 7:S7-18. [Google Scholar]
[3]. Kashyap SR, Defronzo RA., The insulin resistance syndrome: physiological considerationsDiab Vasc Dis Res 2007 4:13-19. [Google Scholar]
[4]. Insulin resistance and menopausehttp://womenshealth.med.monash.edu.au [Google Scholar]
[5]. Catalano D, Trovato GM, Spadaro D, Martines GF, Garufi G., Insulin resistance in post menopausal women: concurrent effects of hormone replacement therapy and coffeeClimateric 2008 11(5):373-82. [Google Scholar]
[6]. Cho LW, Jayagopal V, Kilpatrick ES, Jennings P, Atkin SL., Comparison of Insulin Resistance levels in pre and post-menopausal women, women with polycystic ovarian syndrome and postmenopausal women with Type 2 DiabetesEndocrine Abstracts 2006 11:716 [Google Scholar]
[7]. Mercuro G, Podda A, Pitzalis L, Zoncu S, Mascia M, Melis GB, Evidence of a role of endogenous estrogen in the modulation of autonomic nervous systemAm J Cardiol 2000 85:787-89. [Google Scholar]
[8]. Baron AD, Steinberg HO, Chaker H, Leaming R, Johnson A, Brechtel G., Insulin mediated skeletal muscle vasodilation contributes to both insulin sensitivity and responsiveness in lean humansJ Clin Invest 1995 96:786-92. [Google Scholar]
[9]. Mercuro G, Podda A, Pitzalis L, Zoncu S, Mascia M, Melis GB, Evidence of a role of endogenous estrogen in the modulation of autonomic nervous systemAm J Cardiol 2000 85:787-89. [Google Scholar]
[10]. Durand S, Davis SL, Cui J, Crandall CG., Exogenous nitric oxide inhibits sympathetically mediated vasoconstriction in human skinJ Physiol 2005 January15562(Pt 2):629-34. [Google Scholar]
[11]. Bonora E, Saggiani F, Targher G, Homeostasis Model Assessment closely mirrows the glucose clamp technique in the assessment of insulin sensitivityDiabetes Care 2000 23:23-25. [Google Scholar]
[12]. Castracane VD, Kauffman RP, Controlling PCOS, Part 1: Assessing insulin sensitivityContemporary OG/GYN 2003 Jan1 [Google Scholar]
[13]. Wilson TE, Cui J, Crandall CG, Effect of whole-body and local heating on cutaneous vasoconstrictor responses in humansAutonom Neurosci: Basic Clin 2002 97:122-28. [Google Scholar]
[14]. Williams MJ, Hunter GR, Kekes-Szabo T, Trueth MS, Snyder S, Berland L, Intra-abdominal adipose tissue cut-points related to elevated cardiovascular risk in womenInt J Obes Relat Metab Disord 1996 20(7):613-17. [Google Scholar]
[15]. Rolland YM, Perry HM, 3rdPatrick P, Banks WA, Morley JE, Leptin and adiponectin levels in middle-aged postmenopausal women: associations with lifestyle habits, hormones, and inflammatory markers--a cross-sectional studyMetabolism 2006 55(12):1630-36. [Google Scholar]
[16]. Goodarzi MT, Babaahmadi-Rezaei H, Kadkhodaei-Eliaderani M, Haddadinezhad S., Relationship of serum adiponectin with blood lipids, HbA(1)c, and hs-CRP in type II diabetic postmenopausal womenJ Clin Lab Anal 2007 21(3):197-200. [Google Scholar]
[17]. Petrie John R, Ueda Shinichiro, Webb David J, Elliott Henry L, Connell John MC, Endothelial Nitric Oxide production and insulin sensitivityCirculation 1996 93:1331-33. [Google Scholar]
[18]. McAuley KA, Williams SM, Mann JI, Walker RJ, Lewis-Barned NJ, Temple LA, Diagnosing insulin resistance in the general populationDiabetes Care 2001 24:460-64. [Google Scholar]
[19]. Steinberg HO, Chaker H, Leaming R, Obesity/ insulin resistance is associated with endothelial dysfunctions. Implications for the syndrome of Insulin ResistanceJ Clinical Invest 1996 June197(11):2601-10. [Google Scholar]
[20]. Ferric Carlomagno A, Coassin S, Circulating endothelin-1 levels increase during euglycemichyperinsulinemic clamp in lean NIDDM menDiabetes Care 1995 18:226-33. [Google Scholar]
[21]. Cardillo C, Kilcoyne CM, Nambi SS, Cannon RO, Quon MJ, Panza JA, Vasodilator response to systemic but not to local hyperinsulinemia in the human forearmHypertension 1998 32:740-45. [Google Scholar]
[22]. Cardillo C, Nambi SS, Kilcoyne CM, Choucair WK, Katz A, Quon MJ, Insulin stimulates both endothelin and nitric oxide activity in the human forearmCirculation 1999 100:829-25. [Google Scholar]
[23]. Arcaro G, Cretti A, Balzano S, Lechi A, Muggeo M, Bonora E, Insulin causes endothelial dysfunction in humans. Sites and mechanismsCirculation 2002 105:576-82. [Google Scholar]