Pharmacovigilance deals with the detection, assessment and the prevention of Adverse Drug Reactions (ADRs) [1]. As new drugs are entering the market at a rapid pace, it is imperative to monitor the adverse drug reactions and to report them appropriately to the pharmacovigilance centres, so as to create awareness among the medical fraternity and also among the public, in order to prevent future adverse drug reactions.
An ‘adverse drug reaction’ is defined by the WHO as ‘A response to a drug which is noxious and unintended, and which occurs at doses which are normally used in man for the prophylaxis, diagnosis, or the therapy of a disease, or for the modification of the physiological functions’ [2]. Many minor and major cutaneous adverse drug reactions occur frequently at the doses which are normally used in man. However, these reactions are ignored or they are not reported appropriately to the Pharmacovigilance centres. Hence, this study was aimed in fulfilling the above mentioned lacunae .
There is an immense need for reporting the adverse drug reactions and for a post-marketing surveillance, as the medicines are evaluated for their toxicity only in a limited group of patients before they are marketed. Hospital based adverse drug reaction programs can provide valuable information about the serious problems which are associated with the usage of drugs [3].
The pharmacovigilance activity must be encouraged and implemented in all the fields of medicine in the future, wherever drugs are used for treatment. The Cutaneous Drug Reactions (CDRs) are among the most frequent adverse events in patients who receive drug therapy. CDRs comprise 10 to 20% of the reported ADRs [4]. In this context, we conducted this study in our hospital to evaluate the clinical pattern and the morphology of various cutaneous adverse drug reactions and their causality, severity and preventability.
Methods
The institutional ethics committee approval and an informed consent from all the patients who were included in this study were taken. Out of the 181 patients who attended the Dermatology Outpatients Department of Sree Balaji Medical College Hospital with suspected drug allergy, 59 patients with Cutaneous Drug Reactions (CDRs) were recruited for this observational study from June to December 2011. The detailed history viz., age, sex, the presenting complaint, duration of the illness, type of the drug, duration of the drug intake, past history of the drug allergy and the past history of the illness were recorded. The physical examination which included the morphology of the cutaneous drug reactions and the area of involvement were recorded with the help of the dermatologists. The causality assessment was done by the Naranjo’s Algorithm [5], The preventability was assessed by using the modified Schumock and Thornton scale [6]. The severity of the reactions was graded according to the University of Virginia Health System Adverse Drug Reaction Reporting program criteria as follows [7]:
Mild: A reaction that does not require treatment or prolongation of the hospital stay
Moderate: A reaction that requires treatment and/or a prolonged hospitalization by at least one day
Severe: A reaction that is potentially life-threatening or that which contributes to the death of the patient, that which is permanently disabling, that which requires intensive medical care (including extended hospitalization), or that which results in a congenital anomaly, cancer, or an unintentional overdose.
Each and every finding was analyzed for the frequency of its occurrence and it was presented as percentage by using descriptive statistics.
Results
The findings were analyzed by using descriptive statistics. The mean age of the patients with the cutaneous adverse reactions was 30.58years ± 1.5; range, 4-59 years). Among the recruited patients, 47.5% were males and 52.5% were females. The major presenting complaints were rash (22%), rash with redness (3.4%), rash with intense itching (20.3% ), eruption with pain (23.75%), acne (6.8%), acne with pain and rash (1.7%) each, hyper pigmentation (3.4%), straie (1.7%), patchy lesions (1.7%) and ulcers in the mouth (1.7%).
The duration of the drug intake varied from 0 to 60 days. Most of the cases developed cutaneous reactions while they were taking the drug.
Among the 59 patients, 8.5% had similar cutaneous reactions earlier and 67% had no previous reactions. Past history of systemic illnesses were present among the recruited patients. In that 15.3% were type 2 diabetics, 6.8% had bronchial asthma, 5% had hypertension and 1.7% had epilepsy.
The areas of involvement were the forearms and the legs (20.3% cases), the chest and the abdomen (15.3% cases) , the face alone (8.5% cases), the arms, chest and the face ( 6.8% cases) and the arms and the legs (5.1% cases). About 5.1% of the patients had mucosal involvement and 1.7% of the patients had involvement of the palms and the soles. Mild cutaneous adverse reactions were observed in 5.1% cases and moderate cutaneous adverse reactions were observed in 94.9% cases. No serious Cutaneous Adverse Reactions were observed in our study. The adverse drug event reporting forms for the voluntary reporting of adverse drug events by health care professionals, which were issued by the CDSCO (Central Drugs Standard Control Organization), Directorate general of Health Services, Ministry of Health and Family welfare. Government of India, were downloaded from http://cdsco.nic.in/pharmacovigilance.htm, which were duly filled and sent to the Institute of Pharmacology, Madras Medical College, Chennai. The ADR monitoring centre was under the Pharmacovigilance Programme of India (PVPI).
The various types of cutaneous drug reactions have been shown in [Table/Fig-1] and the commonly implicated drugs have been shown in [Table/Fig-2].
The causality assessment was done by Naranjo’s Algorithm. The severity of the reactions was graded according to the University of Virginia Health System Adverse Drug Reaction Reporting Program criteria, which are given in the [Table/Fig-3,4].
The Cutaneous Adverse Drug Reactions vary in the patterns of their morphology and distribution according to the patterns of the drug intake in that particular area and set up. The studies which were conducted previously in north India showed common morphological patterns such as maculopapular rash (34.6%) , fixed drug eruptions (30%) and urticaria (14%) [8]. A study from South India showed fixed drug eruptions (31.1%) and maculopapular rashes (12.2) [7]. But our present study showed the most common cutaneous drug reactions to be urticaria (32.2%), followed by fixed drug eruptions (25.4%) [Table/Fig-1] acne form eruptions (13.6%), morbilliform eruptions (6.8%), maculopapular rashes (5.1%) and angio-oedema (3.4%) . The differences in the occurrence of the cutaneous adverse drug reactions vary, depending on the frequency of the drug intake [4], the genetics, the associated illness and the immunological basis of the patients.
The various types of cutaneous drug reactions
| Lesion | Frequency | Percent |
|---|
| Acneform eruption | 8 | 13.6 |
| Angioedema | 2 | 3.4 |
| DLE | 1 | 1.7 |
| Drug induced icthyosis | 1 | 1.7 |
| Fixed drug eruption | 15 | 25.4 |
| Irritant contact dermatitis | 1 | 1.7 |
| Lichenoid dermatitis | 1 | 1.7 |
| Maculopapular rash | 3 | 5.1 |
| Morbilliform eruption | 4 | 6.8 |
| Photosensitivity reaction | 1 | 1.7 |
| Pitriasis Rosea | 1 | 1.7 |
| Steroid induced striae | 1 | 1.7 |
| Utricaria | 19 | 32.2 |
| Total | 59 | 100.0 |
The commonly implicated drugs causing CDRs
| Drug | Frequency | Percent |
|---|
| Albendazole | 1 | 1.7 |
| Amoxicillin | 5 | 8.5 |
| Ampicillin | 2 | 3.4 |
| Atorvastatin | 1 | 1.7 |
| Azithromicin | 2 | 3.4 |
| Lignocaine | 1 | 1.7 |
| Native liniments | 1 | 1.7 |
| NSAIDs | 23 | 39.1 |
| Phenytoin | 1 | 1.7 |
| Quinolones | 13 | 22.1 |
| Steroids (Oral) | 5 | 8.5 |
| Steroids (Topical) | 4 | 6.8 |
| Total | 59 | 100.0 |
Probability (Naranjo Algorithms)
| Scale | Inference | Frequency | Percent |
|---|
| 4 | Possible | 1 | 1.7 |
| 5 | Probable | 15 | 25.4 |
| 6 | Probable | 33 | 55.9 |
| 7 | Probable | 9 | 15.3 |
| 8 | Probable | 1 | 1.7 |
| Total | - | 59 | 100 |
Severity assessment (University of Virginia Health System Adverse drug reaction reporting program criteria )
| Assessment | Frequency | Percent |
|---|
| Mild | 3 | 5.1 |
| Moderate | 56 | 94.9 |
| Total | 59 | 100 |
The drugs which commonly produced Cutaneous Drug Reactions CDRs in our study were NSAIDs ( 39.1%), followed by Quinolones (22.1%), Amoxycillin (8.5%), Corticosteroids-oral (8.5%) and topical medications (6.8%) [Table/Fig-2]. This may have been due to the intake of the commonly prescribed NSAIDs for all types of painful conditions and Quinolones were the common antimicrobial agents which were used. Urticaria was seen commonly (32.2%) in our study [Table/Fig-5]. Fixed drug eruptions were observed in a study which was done in South India [7]. Fixed drug eruptions were frequently seen in the patients who took NSAIDs and Quinolones, particularly Ofloxacin [Table/Fig-6,7]. Urticaria was seen in the patients who took NSAIDs. and maculopapular rashes were seen in the patients who took Amoxycillin/Ampicillin antibiotics. One case each of Atorvastatin induced icthyosis, local anaesthetic lignocaine induced local dermatitis [Table/Fig-8] and antiepileptic drug phenytoin induced urticaria were also recorded in our study [Table/Fig-6,7]. Urticaria was seen in the patients who took NSAIDs. and maculopapular rashes were seen in the patients who took Amoxycillin/Ampicillin antibiotics. One case each of Atorvastatin induced icthyosis, local anaesthetic lignocaine induced local dermatitis [Table/Fig-5].
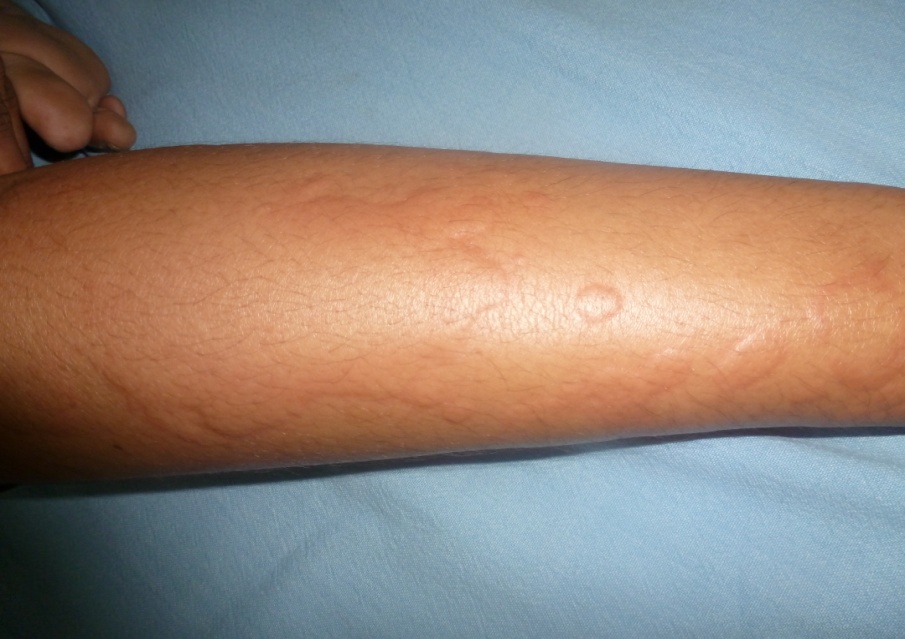
Acute Urticaria due to NSAIDs
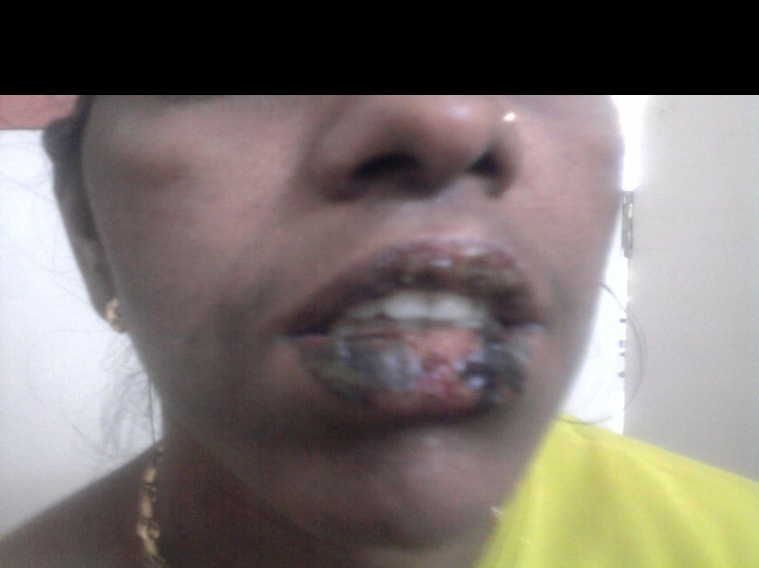
Fixed drug eruption with mucosal involvement due to NSAIDs
There was a predominance of CADRs in the female patients, possibly because women were more concerned about the skin lesions. A majority of our patients belonged to the mean age of 31 years, which was similar to that in a study which was conducted in north India [8]. One more study showed that adults who were aged 20-49 years were at an increased risk of antibiotic related drug eruptions due to the excessive use of antibiotics [9], which was similar to that which was seen in our study. The differences in the findings in many studies may be attributed to the regional variations in the drug intake of the patients and the prescribing patterns.
A history of a previous systemic illness was present in 40.5% of the patients, among which it was diabetes in 15.3% cases, bronchial asthma in 8.8% cases, hypertension and diabetes in 3.45% cases and epilepsy in 1.7% of the patients. The increased incidences of the cutaneous adverse drug reactions in the diabetic patients which were observed in our study, were probably due to the increased incidence of the diabetes and the altered immunological status of the diabetic patients [10].
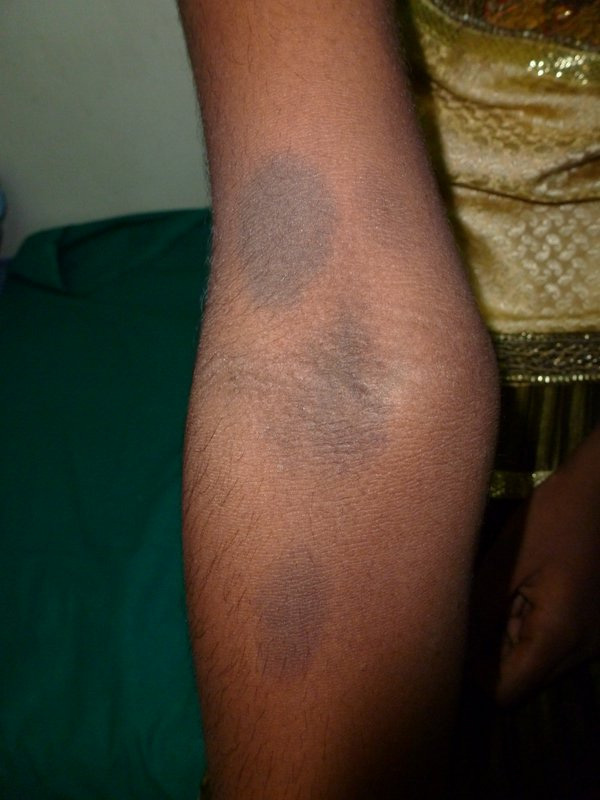
Fixed drug eruption due to Quinolones
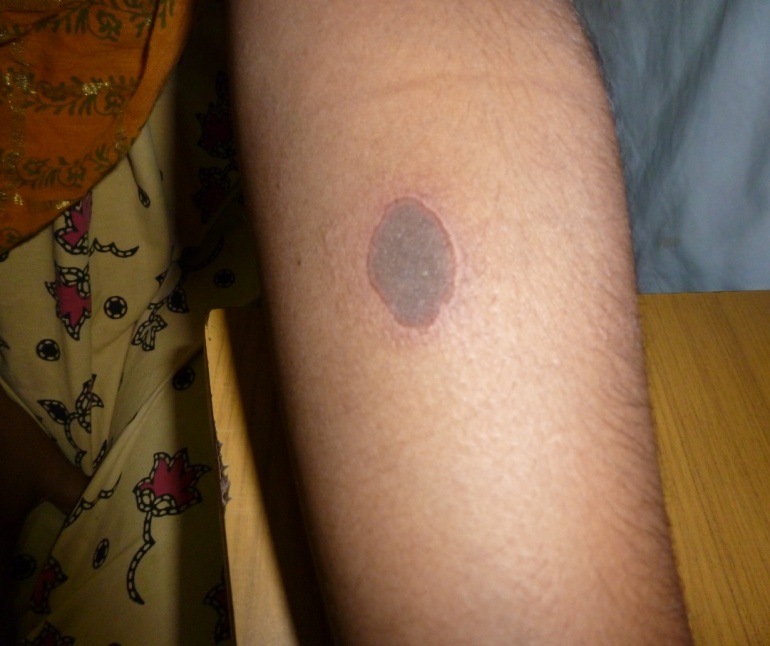
Cutaneous allergy due to Lignocaine test dose
The causality assessment was done by the Naranjo’s Algorithm [5]. According to that, 98.3% of the reactions were probable and 1.7% were possible reactions. This gave a clear evidence that for most of the reactions, the causative agents could be established with the help of the above mentioned scale, that would help in the prevention of the adverse reactions.
The severity of the cutaneous adverse reactions were graded by the University of Virginia Health System Adverse Drug Reaction Reporting Program criteria [7]. According to that, 5.1% of the reactions are mild reactions, whereas 94.9% of the reactions were moderate reactions. Since the study was conducted in the out patients department, the reactions were mild to moderate in nature. The preventability was assessed by the modified Schumock and Thornton scale [6]. To our surprise, all the reactions were found to be preventable. Hence, a careful history taking, and exercising at most caution during prescription of the drugs can prevent most of the adverse drug reactions.
Conclusion
The patterns of the cutaneous adverse drug reactions and the drugs which caused them, varied in our study according to the pattern of the drug intake, the associated illness and the susceptibility of the patients. A sound knowledge of the adverse drugs reactions, a careful history taking and a cautious approach during the prescription of new drugs can prevent most of the adverse drug reactions. As newer drugs are entering our market at a rapid pace, Pharmacovigilance, with special attention to monitoring and reporting of the cutaneous adverse drug reactions must be encouraged.