Introduction
Breast cancer is the most common malignancy in females in the developed countries. The incidence of breast cancer has increased in our country in the recent years. According to the Indian Council of Medical Research (ICMR), there will be a 21% increase of the disease by the year, 2020. The incidence of breast cancer is rising in every country of the world, especially in the developing countries such as India. A family history of breast cancer increases the risk as follows: if a woman has a mother who has suffered from breast cancer, her risk increases to about 3 fold, while having a sister with breast cancer increases the risk to about 2-3 fold. About 5% of the breast cancers are hereditary, i.e. due to a gene being transmitted either from the father or from the mother. Two genes, namely BRCA1 and BRCA2, have been identified although genetic testing and because of the ethical, emotional and the social implications that they carry, they are still in the sphere of research in most of the developed countries except in the U.S.
Thankfully, the incidence of breast cancer is much lower in India as compared to that in the western countries. The incidence varies between the urban and the rural women; the incidence in Mumbai, India, is about 27 new cases per 100,000 women per year, while in the rural Maharashtra state in India, it is only 8 per 100,000. The chances of a cure in the women who develop this disease are directly related to an early diagnosis. In the UK, breast cancer is the most common type of cancer among women. More than 45,500 cases of breast cancer are diagnosed every year, usually in women who are over 50 years of age, who have reached menopause. However, it is possible for the women of any age to be affected by breast cancer and, in rare cases, the condition can affect even men. In 2009, according to the American Cancer Society, 194,280 new cases were diagnosed and 40,610 deaths due to breast cancer occurred in the United States. Hence, it has become important to understand the pathogenesis of this feared epidemic for the treatment, prognosis evaluation and the disease free survival worldwide for women. The treatment modalities in the yesteryears were focused more on radical surgeries, but the latest methods include breast conserving surgeries and implants to facilitate the patients’ cosmetic needs.
Materials and Methods
Histopathology
Hematoxylin-Eosin (Merck, USA) stained sections from 50 formalin-fixed breast cancer specimens which had been obtained in the years, 2008 to 2011, were diagnosed by a single histopathologist to avoid inter-observer variations. The tumours were evaluated for the grades 1-3 by the Nottingham Modification of the Scarff-Bloom-Richardson method, taking into account the following parameters – tubule formation, necrosis and mitotic figures. The mitotic figures were counted at 40x high power by using light microscopy (Olympus U-MDOB3, Japan). The tumours were also evaluated for the histologic type, apoptotic tumour cells, border appearance, lymphocytic stromal response, nucleoli, nuclear chromatin pattern, apocrine features, metaplastic features and the medullary features. The histopathologic features of the luminal-like, Her2+ and the basal-like subtypes were compared. The special tumour types were not graded and their immunohistochemical characteristics were tabulated individually.
Immunohistochemistry
IHC was performed by using the following antibodies – the oestrogen receptor (ER), the progesterone receptor (PR), and the c-erbb2 (Her2neu) receptor, (Biogenex, USA) on FFPE tissues. The antigen retrieval was achieved by using the microwave method with 10mmol citrate buffer at pH 6.0 for 25 minutes. PBS (phosphate buffer saline) was used as the wash buffer and DAB (3,3’ diaminobenzene tetrahydrochloride) was used as the chromogen. The endogenous peroxidise activity was blocked by using hydrogen peroxide. Post protein blocking, the slides were incubated overnight (8 hrs) with the available ER, PR, and the Her2neu primary antibodies and they were conjugated with streptavidin Horse Radish Peroxidise (HRP). The slides were counterstained with hematoxylin, they were examined by light microscopy and the images were captured (Optec APCAM-5, ADELTA, India). In case of the Her2 receptor, 10% staining was the minimum criteria which was required for the tumour immunoreactivity to be considered as positive. The tumours were classified as the luminal-like (ER/PR+,Her2neu -), Her2+ (ER/PR -, Her2neu+) and the triple negative types (ER/PR/Her2neu-).
RNA Extraction and RT-PCR
A BRCA1 cDNA fragment (bp position 390–647, exons 4–6) was amplified by RT-PCR RotorGene 6 Plex (Qiagen, Switzerland) by using the primers 5’- TGTGCTTTTCAGCTTGACACAGG-3’ and 3’-CGTCTTTTGAGGTTGTATCCGCTG-5’ and a Rotor-Gene Multiplex RT-PCR kit® (Qiagen, Germany) against the housekeeping gene, ACTB (Beta Actin).
Results
The mean age of presentation of the tumour was 48.34. In our series, we observed that 36% of the patients were of the age group of 20-40 years, that 46% of the patients were within the age group of 41-60 years and that only 18% of the patients were over 60 years of age. Hence, a majority of the cases lay within the 41-60 years age group. A majority of the tumours (n=23) were seen in the 41-60 year age group and they were mostly high grade tumours (grade 3). This observation was followed by the fact that in the age group of 20-40 years, the grade 1 tumours were more in number. Also, the patients in the over 60 years age group (n=9) mostly had (n=4) high grade tumours. Therefore, of the 50 patients with breast cancer, 23 patients had high grade tumours. Of the special types of breast cancers, papillary carcinomas (37%) were the commonest, followed by an equal distribution (25%) of invasive lobular carcinomas and mucinous carcinomas. Apocrine carcinomas (n=1) accounted for the least of the 4, with an incidence of only 13% in 3 years. Of the 42 cases of breast carcinomas which were graded, 14.29% were grade 1 tumours, 28.57% of them were grade 2 tumours and a majority (57.14%) of them were grade 3 neoplasms. The tabulation of the molecular subtypes of the tumours and their percentages revealed that the tumours were predominantly luminal-like breast cancers (46%), followed by the triple negative (32%) and the Her2neu (22%) subtypes. This proved the vast heterogeneity that the breast cancers exhibited in our series of cases. Although the high grade tumours were the most common ones, the molecular subtype, luminal-like was more prevalent. Hence, the high grade tumours need not always exhibit the triple negative phenotype. The tabulation of the special cases of breast carcinomas and their subtypes, as were evaluated by IHC, showed that the luminal-like breast cancers were predominant and that ILC, which was a high grade tumour, was of the triple negative subtype. A correlation between the grades of the tumours and their molecular subtypes showed that 93% of the high grade tumours were triple negative breast cancers, whereas the luminal-like breast cancers were mostly of the lower grades. This showed that the high grade tumours were almost always associated with hormone receptor negativity, while the low grade breast carcinomas were likely to be hormone receptor positive [Table/Fig-1,2,3,4,5,6,7,8,9].
Number of Cases per Age Group
| S. No | Age Group | No. of Cases |
|---|
| 1 | 20-40 YRS | 18 |
| 2 | 41-60 YRS | 23 |
| 3 | >60 YRS | 9 |
Age Group and Grade of Tumors
| Sl No. | Age Group | No. of Cases | Grade 1 | Grade 2 | Grade 3 |
|---|
| 1 | 20 - 40 YRS | 18 | 6 | 5 | 4 |
| 2 | 41 -60 YRS | 23 | 0 | 7 | 15 |
| 3 | > 60 YRS | 9 | 0 | 1 | 4 |
Special Types of Breast Cancer
| S. No. | Types | No. of Cases |
|---|
| 1 | Papillary Carcinoma | 3 |
| 2 | Mucinous | 2 |
| 3 | Apocrine | 1 |
| 4 | Invasive Lobular Carcinoma | 2 |
Immunohistochemical Classification
| S. No. | Molecular Subtype | No. of Cases |
|---|
| 1 | Luminal | 23 |
| 2 | Her 2+ | 11 |
| 3 | Basal Like | 16 |
Special Types and Molecular Subtype
| S No. | Special Type | No. of Cases | Sub Type |
|---|
| 1 | Papillary | 3 | Luminal |
| 2 | Mucinous | 2 | Luminal |
| 3 | Apocrine | 1 | Luminal |
| 4 | Invasive Lobular Carcinoma | 2 | Basal Like |
Grade and Molecular Subtype of Tumors
| Sl No. | Grade | Subtype |
|---|
| 1 | 1 | 6 - Luminal 0 - Her 2+ 0 - Basal Like |
| 2 | 2 | 6 - Luminal 5 - Her 2+ 1 - Basal Like |
| 3 | 3 | 4 - Luminal 7 - Her 2+ 13 - Basal Like |
Special Types of Breast Cancers And Age Groups
| Sl No. | Special Type | No. of Cases | 20 to 40 Yrs | 41 to 60 Yrs | > 60 Yrs |
|---|
| 1 | Papillary | 3 | 1 | 1 | 1 |
| 2 | Mucinous | 2 | 0 | 0 | 2 |
| 3 | Apocrine | 1 | 1 | 0 | 0 |
| 4 | Invasive Lobular Carcinoma | 2 | 1 | 0 | 1 |
Age Group and Molecular Subtype of Tumors
| Sl No | Age Group | Luminal | Her 2+ | Basal Like |
|---|
| 1 | 20- 40 Yrs | 12 | 2 | 4 |
| 2 | 41-60 Yrs | 8 | 5 | 10 |
| 3 | > 60 Yrs | 3 | 2 | 4 |
Grade, Special Type, Molecular Subtype of Tumors and Qrt-Pcr Values
| Sl No. | Grade and Special Type | Molecular Subtype of Tumors | Qrt-Pcr Values |
|---|
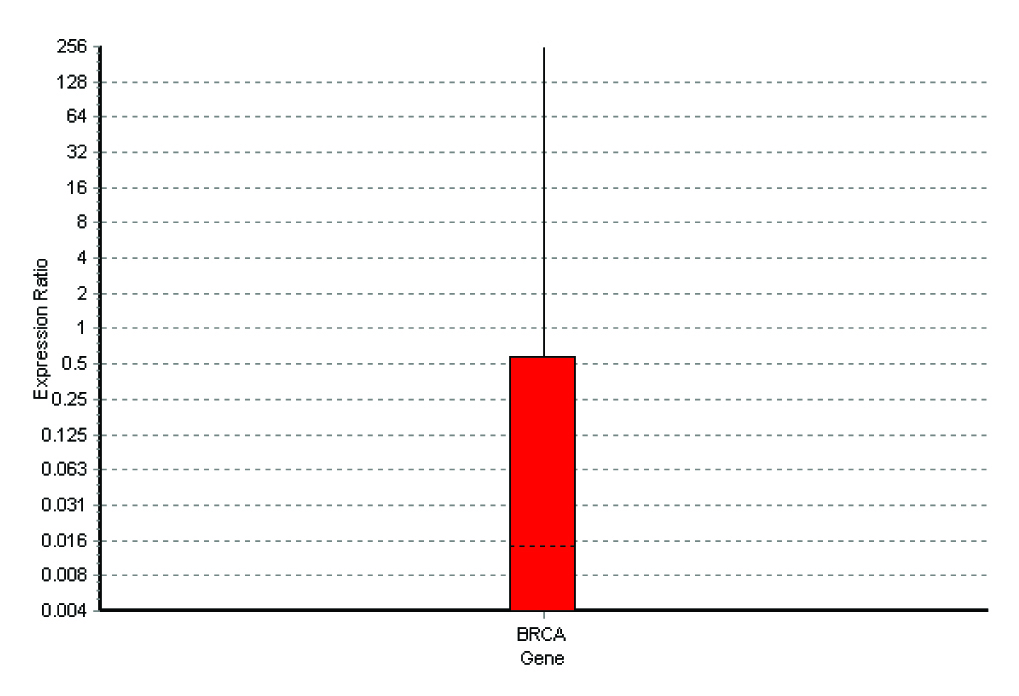
| 1 | Grade-1 | 6 - Luminal | 0.016 |
| 2 | Grade-2 | 6 - Luminal 5 - Her 2+ 1 - Basal Like | 0.5 |
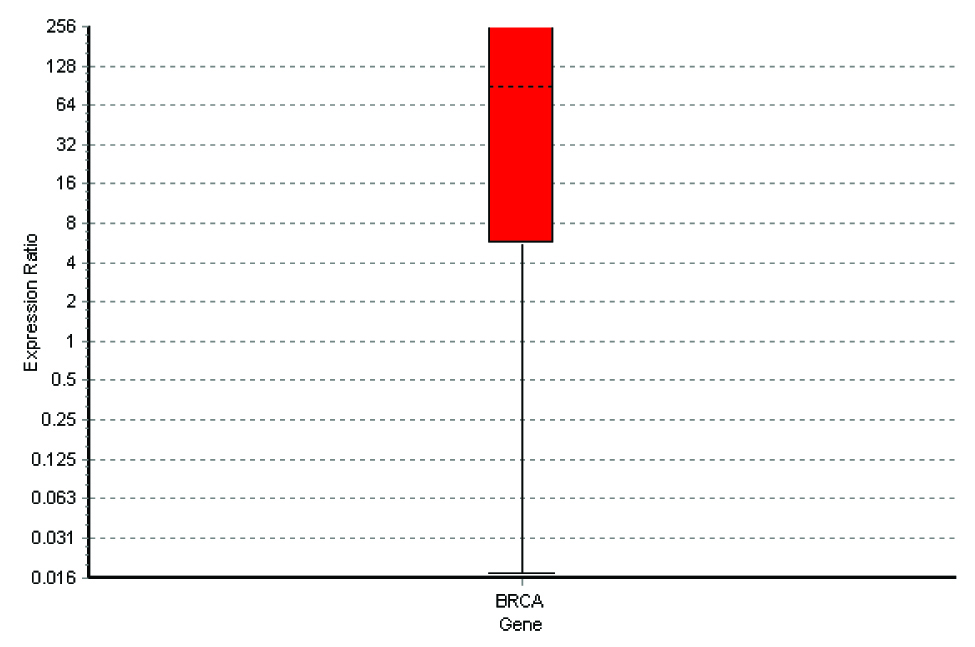
| 3 | Grade-3 | 4 - Luminal 7 - Her 2+ 13 - Basal Like | 72 |
| 4 | Special Type | 6 - Luminal 2 - Basal Like | 65 |
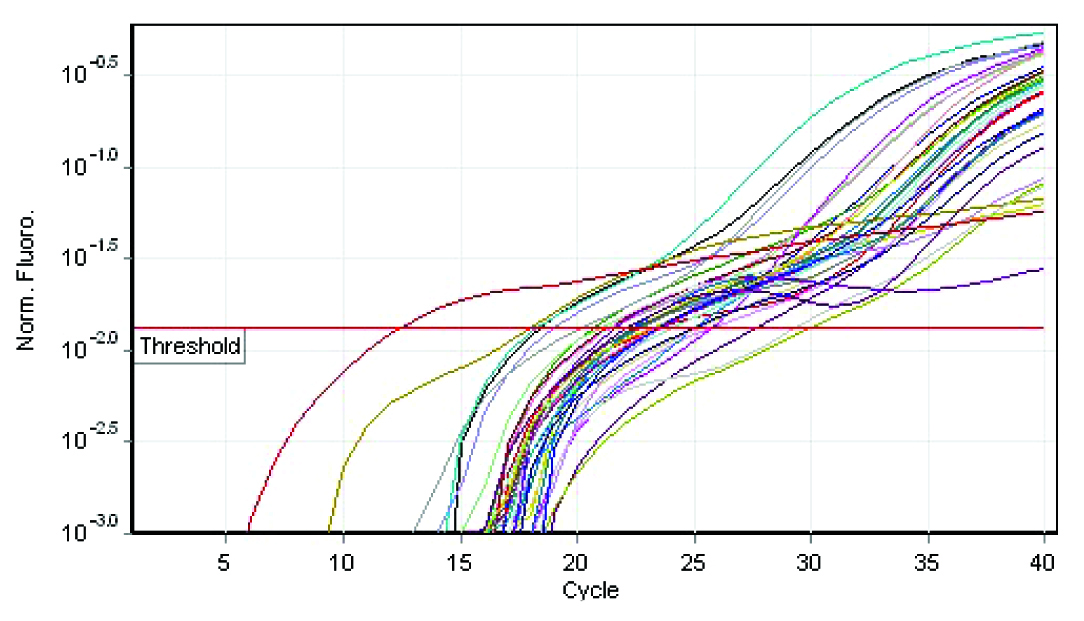
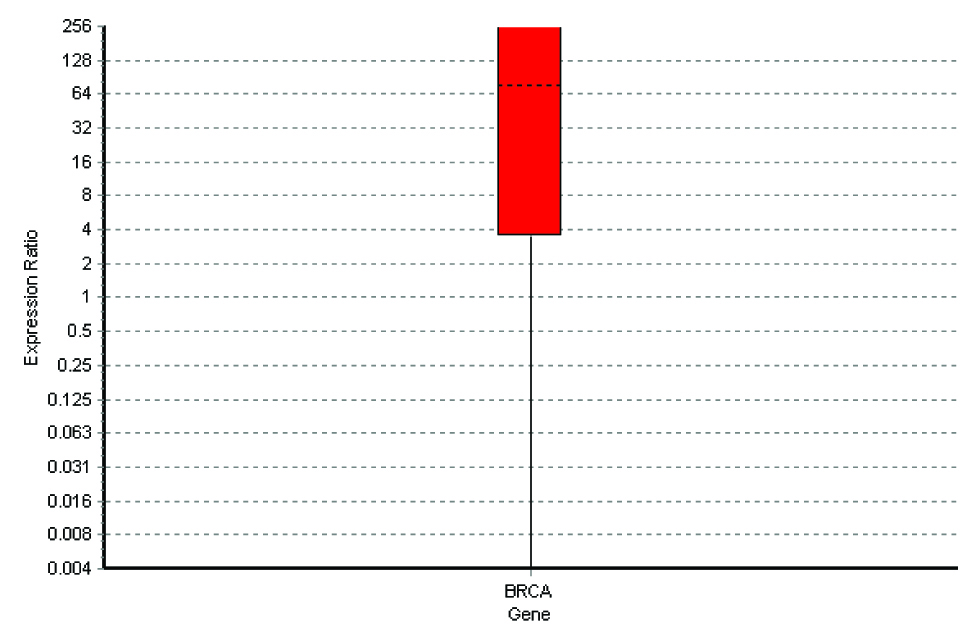
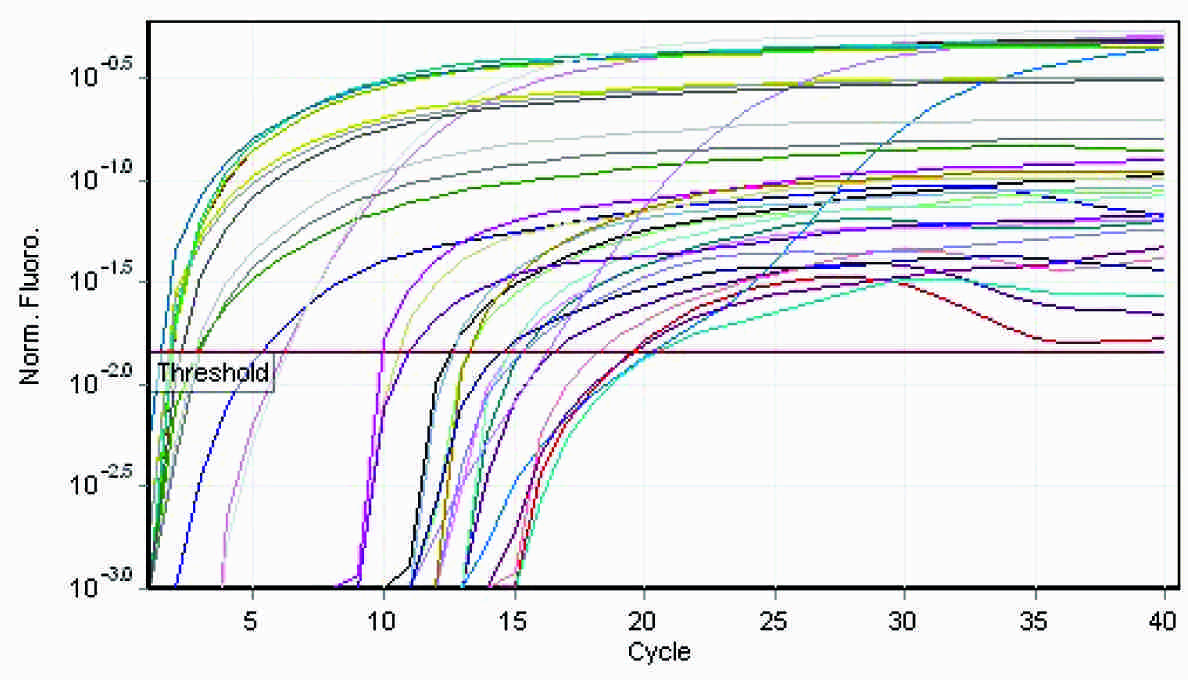
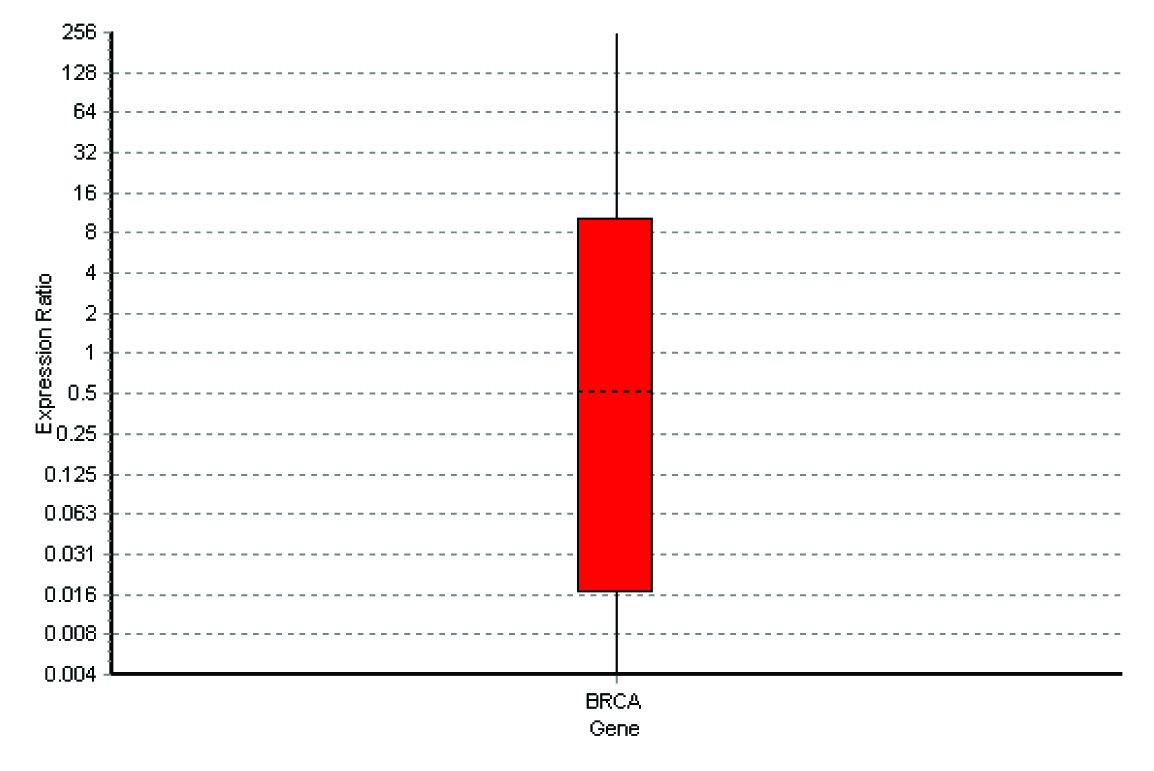
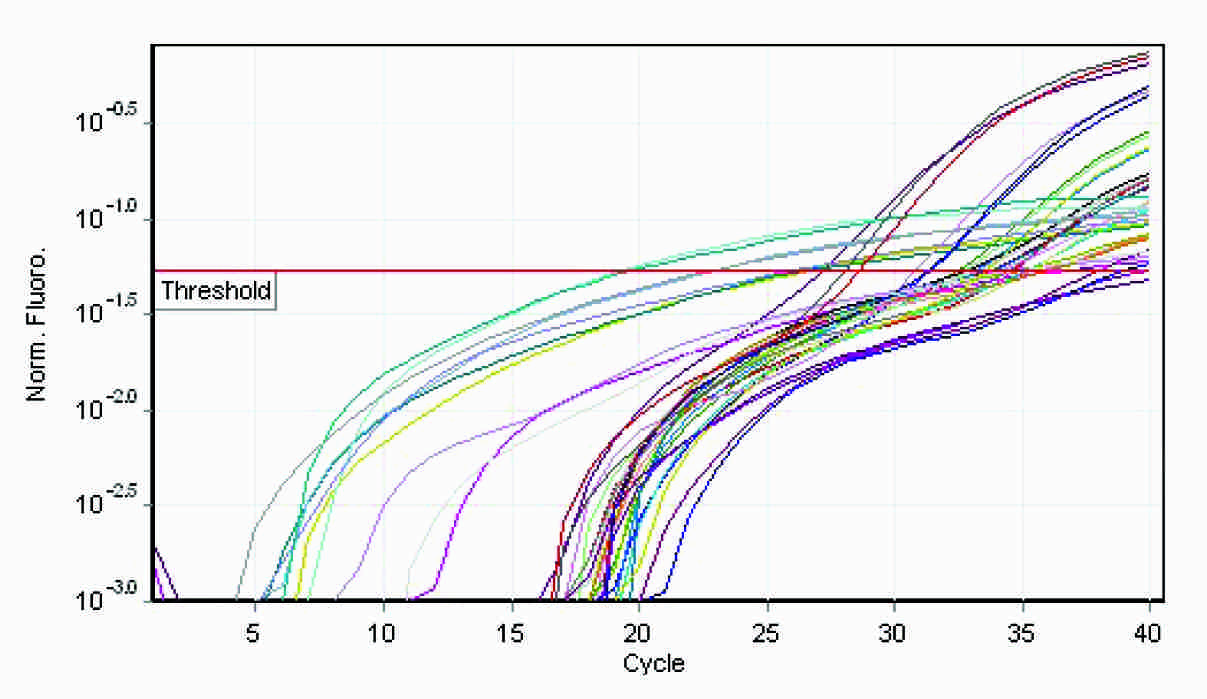
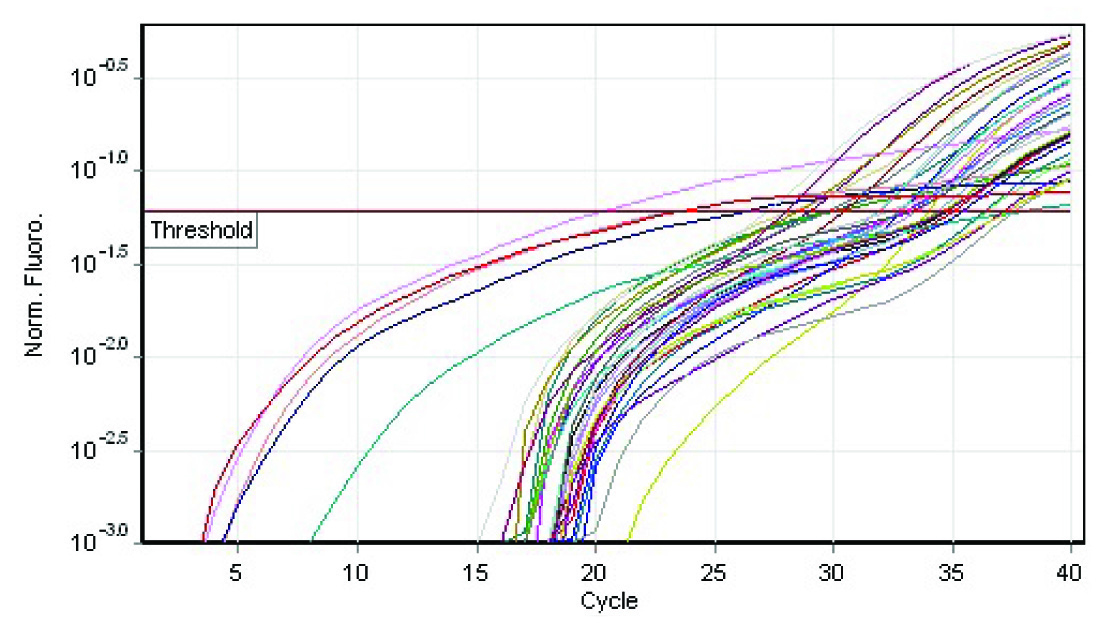
Special types of breast carcinomas RT-PCR
Estrogen Receptor Positivity – IHC ( X 40x)

Progesterone Receptor Positivity – IHC (X 40x)
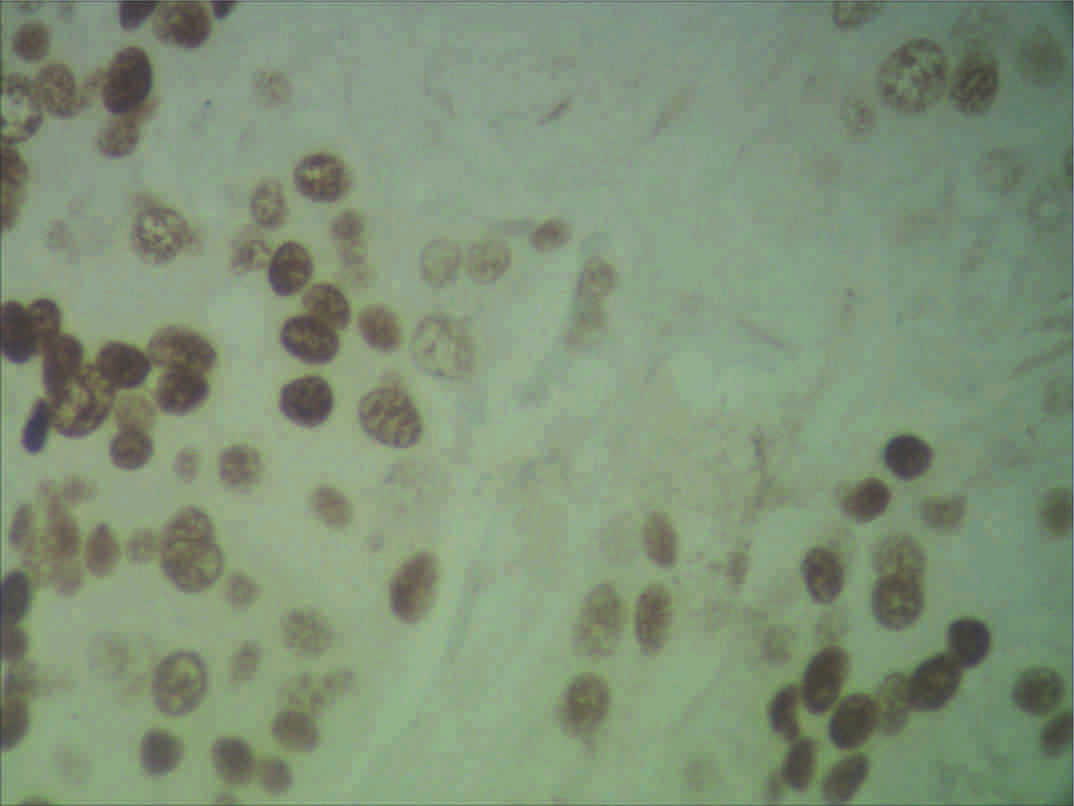
Her2neu Receptor Positivity - IHC (X40x)
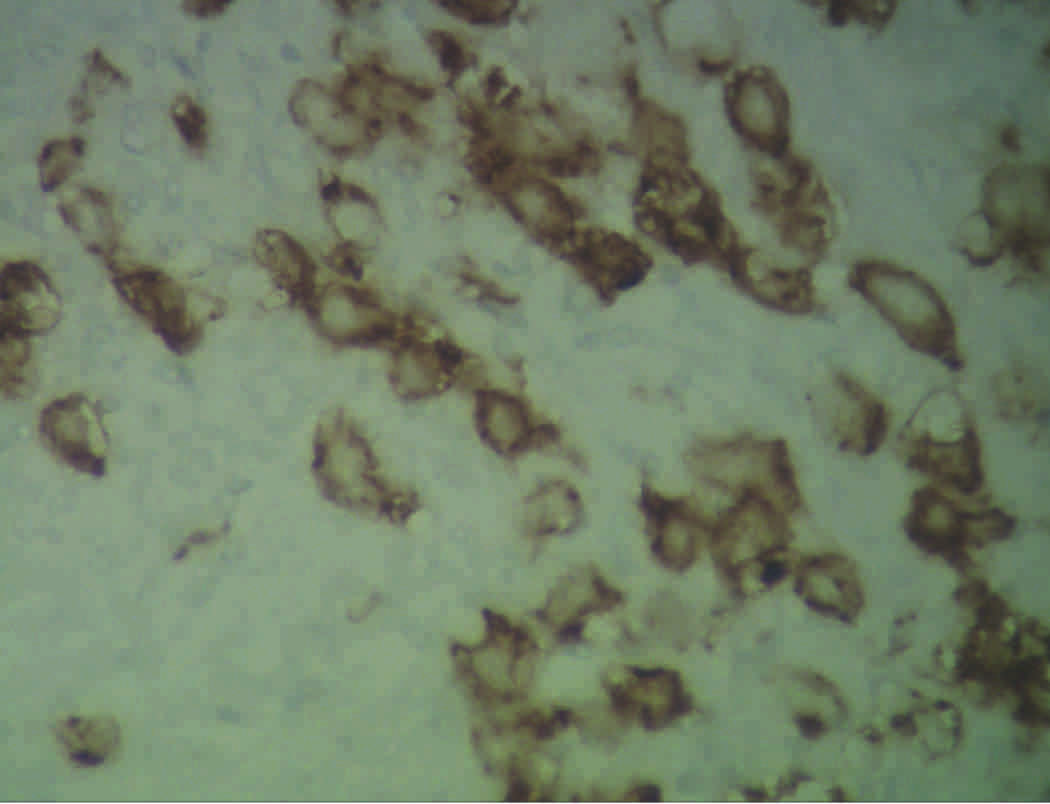
Inflitrating Carcinoma Breast – H&E (X10x)
The q rt-pcr values represented the amount of mRNA of the selected portion of the gene which was present in each sample. From the tabulations, it was evident that higher the grade of the tumour, higher was the quantity of the BRCA1 fragment which was present in the sample. Also, when this was compared with the immunohistochemical profile of the selected tumours, the basal-like subtype was more frequently observed among the grade 3 tumours, with the highest of the q rt-pcr values. In our case series, a correlation could thus be established between the tumour grades, the hormone receptor positivity and the quantification of the BRCA1 gene.
From the four graphs and the boxplot figures, it is clear that higher the grade of the tumour, more severe is the level of expression of the BRCA1 gene. The same applies to the special types of the breast carcinomas which are in question also.
Discussion
[Table/Fig-11,12,13,14,15,16 & 17].
Breast cancers are of a wide variety and they exhibit several characteristics which form the important factors in their diagnosis and treatment. Hence, it is imperative to recognize all the possible variables of breast cancers, which include the age of the patients at incidence, grades of the cancers and their immunohistochemical and genetic profiles.
Age is an important factor in the diagnosis of breast cancer. Advancing age increases the risk of development of breast cancers [1]. The youngest age at presentation here was 28 years and the oldest was 70 years. It has been estimated that within the next ten years, 1 out of every 8 women in the world have the probability of developing breast cancer in their lifetime [2,3].
The Immunohistochemical Profile - A number of studies compared the oestrogen and the progesterone receptor status between the primary and the metastatic carcinomas to the corresponding lymph nodes [4,5]. In 2003, Iguchi and colleagues evaluated 87 patients for the oestrogen receptor expression in primary and metastatic carcinomas to the corresponding lymph nodes. Their study showed a 75.9% concordance between the primary tumour and the metastases. 23% cases were ER positive and 52.9% were negative, both in the primary and the metastatic tumours. The ER expression differed in 24.1% cases. In 1995, Nedergaard L evaluated 101 patients. The ER status in the primary and the metastatic tumours was concordant in 79% patients and it was discordant in 21% cases. In 1983, Holdaway showed a 46% concordance between the primary and the secondary tumours for the oestrogen and the progesterone receptors. Mori and Morimoto, in 1991, showed the decreased expression and the intensity of the oestrogen receptors in the metastases to the lymph nodes. Cianga C showed that 30% breast carcinomas had lost their expression of the hormone receptors from the primary tumours to the axillary metastases. However, the presence of ER is no warrant for the response to the endocrine therapy because 40 to 50% patients with ER positive primary tumours do not respond and almost all the patients ultimately develop metastases which are refractory to the treatment [6]. About 10% of the ER negative carcinomas are hormone dependent. Many mechanisms may potentially be responsible for these controversial observations.
The oestrogen and the progesterone receptor positive primary tumours contain a small percentage of cells which do not express hormone receptors. These cells represent the poorly differentiated population of cells which have the potential to metastasize to distant sites and they usually do not respond to the endocrine therapy [4]. The HER-2/neu proto-oncogene is overexpressed in 25% to 30% of the human breast cancers and it is usually associated with the tumour aggressiveness and a poor prognosis [7,8]. However, in a great majority of the cases, the HER-2/neu status is determined on the basis of the primary tumour [7]. Studies have reported a high level of consistency, although they were not complete, in HER-2/neu between the primary and the loco-regional metastases by using immunohisochemistry [4]. In 1999, Tuziak and Olszewski evaluated 71 cases of breast cancer. They found the HER-2/neu over expression in 40.8% primary and 40.9% metastatic tumours. A study which was done by Tanner and Jarvinen showed amplification in 28% of the primary tumours and it was also always associated with an amplification in their metastases.
According to the studies which were conducted by Hoff et al., regarding the immunohistochemical characteristics of breast tumors, it was observed that the hormone receptor negativity was associated with an incidence of the tumour in an older age group and a poorer outcome. Also, observations which were made by Nidal et al. (2005) showed that the Her2 amplification was associated with a negative hormone receptor status and that it was almost always associated with a higher tumour grade, a larger size of the tumour and a high rate of lymph node metastases. RA Walker and colleagues (2007) of the University of Leicester, UK, postulated that immunohistochemical markers could be predictive tools in breast cancers. They evaluated various antibodies such as ER, Her2, Ki67, p53 and others to arrive at a conclusion that ER was the single most important predictor of the treatment response for breast cancer and that the newer markers would require further evaluation and standardization before being employed in the patient care. Their study composed of the analysis of several other factors which included the tissue fixation, processing, scoring and the assessment of the immunohistochemical results and also the inter-observer errors. The latest studies have shown that the identification of the Her2 amplification by FISH was more effective than its detection by IHC [8].
The BRCA1 Gene Rearrangements
In the present study, all the fifty cases showed amplification of the selected fragment of the gene, thus implying that the frequency of occurrence of the BRCA1 gene re-arrangements in the selected population from the geographical region was 100%. This proves that gene mutations may arise de novo in the patients with breast cancer and that they may be considered as the individual prognostic markers for the patients. The BRCA1 gene mutations have been documented in Indian breast cancer patients [9,10]. According to Saxena et al., a study which was conducted at Safdarjung Hospital, New Delhi, showed that two novel splice variants existed in 10% of their breast cancer patients.
The counterpart of the BRCA1 gene, the BRCA2, warrants further analysis to determine as to whether the mutations in the latter may co-exist with the former in causing the disease process or in influencing the prognosis in breast cancers [11].
Stephen et al., in 1997, found that 1% of the Ashkenazi Jewish population carried the 185delAG mutation of the BRCA1 gene [12]. In their study, the second most common mutation in the same population was 5382insC and approximately 20% of the patients who were diagnosed before the age of 40 years possessed the 185delAG mutation. Also, they observed that the ductal histologic type of breast carcinoma was mostly associated with the BRCA1 mutations in comparison with the tumours with medullary features. According to the observations of Nancy et al (Algeria, 2008), the Algerian patients commonly carried six deletions or duplications of the nucleotides 1 to 29 of exon 5. The mutation which was documented was c.798_799delTT. It is the first non-Jewish mutation to be described in the literature. The Algerian patients were noted to have larger tumour sizes when they were associated with the BRCA1 mutations. However, in contrast, the non-mutation cases also showed a higher nuclear grade and oestrogen receptor negativity with lymph node positivity. This diversity seems to exist among all the studies which have been reviewed in the literature [13].
Gayther et al., [1] reported the BRCA1 mutations, 1080delT, 1129delA, 2371-2372delTG and 3976-3979delGTGA (clustered 50bp from each other) in the Chinese population amongst the patients in the age group of 43 to 64 years. In another population based study which was conducted by Zhen et al (2003) in China, they discovered that the prevalence of the patients with early onset breast cancer was 9.1%, the mean age at diagnosis being 33 years. Four BRCA1 gene mutations were identified (589delCT, IVS7-24delACTGTTCTTT, Val191Ile and 1081delG) [14,15]. From the present case series, it was evident that the BRCA1 gene re-arrangements had influenced the tumour development in all the selected fifty patients and that it was an independent factor which determined the same. The age group, grade or the immunohistochemical characteristics did not seem to influence the BRCA1 status of the patients. However, a significant correlation can be arrived at, when the results of the grades of the tumours, their hormone receptor statuses and BRCA1 gene re-arrangements are analyzed. The exact cause of the occurrence of this series of findings has not yet been established.
Conclusion
To the best of our knowledge, this is one of the few systematic studies which have been done in our country, with a special reference to the BRCA1 gene re-arrangements in a specific geographical location. The aim of this study was to conduct useful research to arrive at a correlation between the various factors that determined and explained breast cancers. The variables which were taken into account were the patients’ age at onset of the tumours and their histological grades, hormone receptor statuses and BRCA1 gene re-arrangements. It was found that the mean age at which the tumour occurred was in the middle age group. The tumours which were investigated in our series were predominantly hormone receptor positive but the high grade tumours were more common. However, the results of the BRCA1 gene re-arrangement analysis revealed that all the fifty patients had BRCA1 mutations of the selected fragment of the gene, which was an alarming finding. The above observations and results showed correlation with those of the studies which were performed by various authors and researchers throughout several institutions around the globe. The present study emphasized the fact that a more extensive research was warranted and that a detailed investigation was to be performed on the various aspects of the tumours, such as the immunohistochemical profile, by using several antibodies and the BRCA1 gene mutations, by using other exon fragments. This would reveal newer findings which could be important in determining the prognosis of the tumours, so that the therapy could be targeted and tailor-made for the patients to increase their disease free survival. Thus, the future of the breast cancer detection and analysis should involve an extensive screening of the patients and their families, which should include their genetic profile, to detect the risk of development of the disease [16].
[1]. Murthy NS, Chaudhry K, Nadayil D, Agarwal UK, Saxena S, The Changing Trends of Breast Cancer : the Indian Scenario. Indian Journal Of Cancer National Cancer Registry Programme (ICMR), Division of Non-Communicable Diseases, Indian Council of Medical ResearchIndian Journal of Cancer 2009 46:173-77. [Google Scholar]
[2]. Hellman Samuel, Dogma and Inquisition in MedicineCancer 1993 71(1):2430-33. [Google Scholar]
[3]. Olson James, Bathsheba’s Breast: Women, Cancer, and History 2002 BaltimoreJohn Hopkins Press [Google Scholar]
[4]. Zafrani B, Aubriot MH, Mouret E, The high sensitivity and the specificity of immunohistochemistry in the detection of the hormone receptors in breast carcinoma: comparison with the biochemical determination in a prospective study of 793 casesHistopathology 2000 37:536-45. [Google Scholar]
[5]. Taucher P, Rudas M, Mader RM, Do we need HER-2/neu testing for all the patients with primary breast carcinomas?Cancer 2003 98:2547-53. [Google Scholar]
[6]. Ellis IO, Schnitt SJ, Sastre-Garau X, Tavassoli FA, Sevilee P, Invasive breast carcinomaPathology and Genetics of Tumours of the Breast and Female Genital Organs 2003 Lyon, FranceIARC Press:13-59.World Health Organization Classification of Tumours [Google Scholar]
[7]. Press MF, Hung G, Godolphin W, The sensitivity of the HER-2/neu antibodies in archival tissue samples: a potential source of error in the immunohistochemical studies of the oncogene expressionCancer Res 1994 54:2771-77. [Google Scholar]
[8]. Miller K, The breast HER2 moduleImmunocytochemistry 2006 5:66-70. [Google Scholar]
[9]. Hall JM, Lee MK, Newman B, Morrow JE, Anderson LA, Huey B, King MC, The linkage of early-onset familial breast cancer to chromosome 17q21Science 1990 December250(4988):1684-89. [Google Scholar]
[10]. Starita LM, Parvin JD, The multiple nuclear functions of BRCA1: transcription, ubiquitination and DNA repairCurrent Opinion in Cell Biology 2003 15(3):345-50. [Google Scholar]
[11]. Paterson JW, BRCA1: a review of the structure and the putative functionsDis. Markers 1998 February13(4):261-74. [Google Scholar]
[12]. Mazoyer S, The genomic rearrangements in the BRCA1 and the BRCA2 genesHum Mutat 2005 25(5):415-22. [Google Scholar]
[13]. Casilli F, The rapid detection of the novel BRCA1 rearrangements in high-risk breast-ovarian cancer families by doing multiplex PCR of the short fluorescent fragmentsHum Mutat 2002 20(3):218-26. [Google Scholar]
[14]. Barrois M, A real-time PCR-based gene dosage assay for detecting the BRCA1 rearrangements in breast-ovarian cancer familiesClin Genet 2004 65(2):131-36. [Google Scholar]
[15]. Narod SA, Ford D, Devilee P, Barkardottir RB, Lynch HT, Smith SA, Ponder BAJ, An evaluation of the genetic heterogeneity in 145 breast-ovarian cancer familiesAm J Hum Genet 1994 56:254-64. [Google Scholar]
[16]. Johannsson OT, Idvall I, Anderson C, Borg A, Barkardottir RB, Egilsson V, Olsson H, The tumour biological features of BRCA1-induced breast and ovarian cancersEur J Cancer 1997 33(3):362-71. [Google Scholar]