The Oxidative Stress in Cataract Patients
Jaskiran Kaur1, Sahiba Kukreja2, Amandeep Kaur3, Naresh Malhotra4, Ravneet Kaur5
1 Assistant Professor, Department of Biochemistry, Sri Guru Ram Das Institute of Medical Sciences and Research, Amritsar, India
2 Associate Professor, Department of Biochemistry, Sri Guru Ram Das Institute of Medical Sciences and Research, Amritsar, India
3 Assistant Professor, Department of Biochemistry, Sri Guru Ram Das Institute of Medical Sciences and Research, Amritsar, India
4 Professor, Department of Biochemistry, Sri Guru Ram Das Institute of Medical Sciences and Research, Amritsar, India
5 Assistant Professor, Department of Preventive and Social Medicine, PGI, Chandigarh, India
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Jaskiran Kaur,Assistant Professor, Department of Biochemistry, Flat no. 2, Registrar flats. Government Medical College campus, Circular Road Amritsar, India.
Phone: 9855707180
E-mail: drjaskiran@rediffmail.com
Background
The recent studies on cataract formation focus on the primary role of the systemic oxidative stress which is generated outside the lens. Our research was directed to assess the oxidative stress by measuring the lipid peroxidation products in the form of the Thiobarbituric Acid Reactive Substances (TBARS) and the antioxidant enzyme levels in the blood. The antioxidant therapy may have a role to play in delaying the onset and the progression of age related cataracts.
Material and Method
This was a case control study. It comprised of 100 age matched subjects (50 with cataracts and 50 controls) with their ages ranging from 45- 75 years. Oxidative stresses such as the Thiobarbituric Acid Reactive Substances (TBARS) and the antioxidant enzymes, Superoxide Dismutase (SOD) and Glutathione Peroxidase( GPX ) were investigated in all the patients and the controls.
Results
Significantly increased levels of serum lipid peroxide in the form of Malondialdehyde (MDA) (p<0.001) were observed in the cataract patients as compared to the controls. Significantly decreased blood levels of SOD and GPX were observed in all the patients.
Conclusion
In the present study, it was concluded that oxidative stress plays an important role in the onset and the progression of cataracts. The pro-oxidant i.e. serum malondialdehyde (MDA) levels were increased in the cataract patients. The blood levels of the enzymatic anti-oxidants, SOD and GPX were decreased. The plasma TBARS can be used as biomarkers of the degeneration in the lens.
Cataract age related, Plasma TBARS, Oxidative stress, Superoxide dismutase, Glutathione peroxidase, Lipid peroxidation(LPO)
Introduction
Cataract is a complete or a partial opacification of sufficient severity on or in the human lens or in the capsule, which impairs the vision. It is one of the leading causes of reversible blindness in the world today. The pathophysiology behind the age related cataracts is complex and it has yet to be fully understood. It is believed that oxidation is a very early or initial event in the overall process in the sequence of events which lead to cataracts [1,2].
J.J Harding proposed a variety of factors which were implicated in the maturity onset of cataractogenesis: a low antioxidant defence capacity, high lipid peroxidation, an augmented non enzymatic glycosylation, a reduced chaperone function of the alpha crystallins and an increased permeability of the lens membrane [3,4].
The lipid peroxidation represents the oxidative tissue damage which is caused by hydrogen peroxide, the superoxide anions and the hydroxyl radicals, which results in the structural alteration of the membrane, with the release of the cell and the organelle contents and the loss of the essential fatty acids, with the formation of cytosolic aldehyde and peroxide products. Malondialdehyde (MDA) is the major end product of the free radical reaction on the membrane fatty acids [5].
SOD is an enzymatic antioxidant which provides defence, that acts by quenching O2 and converting it into H2O2. This protects the cell membrane from the damage which is caused by the Reactive Oxygen Species ( ROS). But the decreased SOD levels may lead to increased lipid peroxidation, resulting in cellular rigidity and deformability [6]. An altered activity of SOD in the cataract patients has been revealed recently [3].
Glutathione Peroxidase (GPX) scavenges the highly reactive lipid hydroperoxide in the aqueous phase of the cell membrane. During aging, the lens loses its antioxidant potencies such as may be seen with the decrease of glutathione or the expression of the antioxidant enzymes [7].
Researchers have shown that the oxidative stress which is caused due to the accumulation of free radicals plays a role in the pathogenesis of cataracts and that this process can be prevented or ameliorated by antioxidants. Studies on the antioxidant status of the lens and the blood in cataract patients have been extensively reported. However, very few studies have been conducted on the Indian patients with cataracts. In the developing countries like India, cataracts evolve earlier in life and they are 3 times more prevalent than those in the developed countries [8]. The present study invites attention to the possible role of the Reactive Oxygen Species (ROS) in the progression of cataracts after estimating the levels of the antioxidant enzymes, SOD and GPX in the blood. The lipid peroxidation product, Malondialdehyde (MDA) was also estimated.
Materials and methods
Fifty patients with cataracts, who were in the age range of 45-75 years, who presented to the Outpatients Department of Ram Lal Eye hospital which is attached to Govt. Medical College, Amritsar, India, were included in the study. Before the start of the study, the approval of the institutional ethical committee was obtained.
A group of 50 normal healthy individuals who were age matched, who were from the same population, served as the controls. Clinical and biochemical research was carried out on all the subjects. During the selection of the subjects from both the groups, it was made sure that they were without a previous medical history of any chronic disease or metabolic disorder. A diagnosis of cataract was established by an ophthalmologist after doing a complete ocular examination. The patients who took any antioxidant drugs were excluded from the study.
After obtaining a written informed consent, venous blood was collected from the subjects under aseptic conditions by venipuncture by using a 10ml sterile disposable syringe and a needle. The serum was separated by centrifugation at 3000rpm for 10 minutes at room temperature. 3ml of whole blood was collected in a heparinized vial for the estimation of the GPX levels in the whole blood. The samples were stored at 4ºC before analysis and all the samples were analyzed on the same day of the collection.
The serum lipid peroxide levels were measured by precipitating the lipoproteins with trichloroacetic acid and boiling them with thiobarbituric acid, which reacted with malondialdehyde to form a pink colour, as per the ‘Kei satoh’ method [9]. The resulting chromogen was extracted with n-butyl alcohol and the absorbance of the organic phase was determined at the wavelength of 530nm. The determined values were expressed in terms of malondialdehyde in nmol/ml.
Serum Superoxide Dismutase (SOD) was analyzed by applying the method of Marklund and Marklund (1974) which was modified by Nandi and Chatterjee in 1988 [10]. Glutathione peroxidase was estimated by the method of Paglia and Valentine 11 by using Ransel-Randox reagent kits which were manufactured by Randox Labs. Ltd. (Crumlin UK).
All the results were expressed as mean± SD. The statistical analysis was done by using the Student’s t’ test. The P values which were <0.001 was considered as highly significant.
Results
The present study was conducted on 50 cataract patients who were aged 45-75 years. 50 age matched healthy individuals served as the controls. The estimated mean serum MDA levels in the cataract patients and the controls were 5.43 ±1.69 nmol/ml and 2.42 ±0.46 nmol/ml.The serum lipid peroxide concentration in the form of MDA was significantly higher in the cataract patients (p< 0.001) as compared to that in the controls [Table/Fig-1]. [Table/Fig-2] shows that the mean serum concentration of superoxide dismutase in the cataract patients and in the control group was 2.75± 0.40 units/ml and 4.25± 1.20 units/ml respectively. The level of the antioxidant enzyme, SOD was significantly decreased in the cataract patients as compared to that in the controls.
Comparison of Serum MDA Levels in Controls and Patients Under Study
| Subjects | No. of cases(n) | Range (nmol/ml) | Mean | ±SD | S.E |
|---|
| Controls | 50 | 1.5-2.8 | 2.42 | 0.46 | 0.07 |
| Patients | 50 | 2.3-7.5 | 5.43 | 1.69 | 0.24 |
Comparison of Serum Sod Levels in Controls and Cataract Patients Under Study
| Subjects | No. of cases | Range units/ml | Mean | ±SD | S.E |
|---|
| Controls | 50 | 2.8-6.66 | 4.25 | 1.20 | 0.17 |
| Patients | 50 | 2.0-3.3 | 2.75 | 0.40 | 0.06 |
The mean glutathione peroxide level in the blood of the cataract patients was 26.23± 11.90. The blood level of GPX in the controls was 70.29± 10.53.The blood levels of GPX were decreased in the cataract patients as compared to those in the control group. The decreases were statistically significant [Table/Fig-3].
Comparison of Blood Glutathione Peroxidase Levels In Controls and Patients Under Study
| Subjects | No. of cases | Range units/gm Hb | Mean | ±SD | S.E |
|---|
| Control | 50 | 42.05-93.46 | 70.29 | 10.53 | 1.49 |
| Patients | 50 | 12.93-45.83 | 26.23 | 11.90 | 1.68 |
Discussion
Cataract is the leading cause of blindness, accounting for 50% of the blindness cases worldwide. Although significant progress has been made towards identifying the risk factors for cataract, there is no proven primary prevention or medical treatment for it. The surgical removal of the cataract remains the only therapy.
The ocular lens which is continually exposed to light and ambient oxygen, is at a high risk of photoxidative damage which results in a cataract. The oxygen free radicals appear to impair not only the lens crystallins which aggregate and precipitate, forming opacities, but also the proteolytic enzymes whose function is to eliminate the damaged proteins. Apart from an enzymatic defence system which consists of superoxide dismutase, catalase and glutathione peroxidase against the excited oxygen species, the lens contains the antioxidants vitamins C and E and presumably beta-carotene as another line of defence [11].
The pathophysiology behind the age related cataracts is complex and it has yet to be fully understood. It is believed that oxidation is a very early or initiating event in the overall process in the sequence of events which lead to the formation of cataracts [12-14]. Oxidative stress may result from an imbalance between the production of the reactive oxygen species and the cellular antioxidant defence mechanisms. In the cells of the eyes, the reactive oxygen species may initiate a surge of toxic biochemical reactions such as peroxidation of the membrane lipids and extensive damage of the proteins, which cause intracellular protein aggregation and precipitation [15].
Lipid peroxidation represents the oxidative tissue damage which is caused by hydrogen peroxide, the superoxide anions and the hydroxyl radicals, resulting in a structural alteration of the membrane, with the release of the cell and the organelle contents and the loss of cytosolic aldehyde and peroxide products. Malenaldehyde is a major end product of the free radical reaction on the membrane fatty acids.
In our study, an increase in the MDA level [Table/Fig-1] was seen, which indicated an increase in the oxidative stress or a decrease in the antioxidant defence mechanism. In the cases of the development of age related cataracts, LPO may also be the real cause of destruction of the plasma membrane of the lenticular fibres and the subsequent oligomerization of the crystalline lens [16]. The lipid peroxidation may be linked to the premature development of senile cataracts [17]. Therefore, it can be stated that LPO is one of the possible causes of the cataract progression.
SOD is an enzymatic antioxidant while provides the first line of defence that acts by quenching O2 and converting it into H2O2. There may be two reasons for the lowering of the SOD levels:
As more and more ROS like O2 are produced, SOD will be used up in the process when it converts O2 to H2O2.
H2O2 also causes inhibition of the SOD activity. There are several classes of SOD that differ in their metal binding ability, their distribution in the different cell compartments and in their sensitivity to various reagents.
Among these, the Cu and the Zn superoxide dismutase SOD1 is widely distributed and it comprises 90% of the total SOD. This ubiquitous enzyme, which requires Cu and Zn for its activity has a great physiological significance and a therapeutic potential.
SOD removes O2 by catalyzing a dismutation reaction which involves the oxidation of one O2 to oxygen and the reduction of another O2 to hydrogen peroxide.
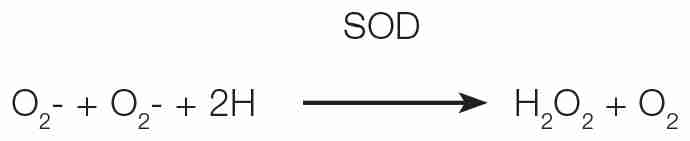
The discovery of SOD led to the realization that the which was formed in vivo in the living organisms and SOD removes (Halliwell, 1991).
Chinese researchers found the lower activities of several RBC antioxidant enzymes (SOD and catalase) and a significantly decreased erythrocyte GPX level in the subjects with the senile lens changes [18]. The POLA study showed a strong association of the high levels of the erythrocyte SOD with an increased risk of nuclear cataracts [19]. In our study, a significant decrease in the serum SOD level (P< 0.001) was observed in the cataract patients as compared to that in the controls [Table/Fig-2].
The GSH antioxidant system is the body’s powerhouse for diffusing and disposing the radicals that threaten the cell and tissue and cause organ damage, thus slowing the approach of age [20]. One of the several possibilities for the occurrence of a lower GSH conc. in the blood is an increased GSH consumption for the removal of peroxides and xenobiotics [21].
GSH is the obvious compound which defends the lens against oxidative insults, being directly involved in reducing the disulfides, being a pivotal cofactor in the detoxication of H2O2 and acting as a free radical quencher.
The present study also indicated an age related decrease in the glutathione peroxidase activity [Table/Fig-3].
Our results confirmed some previous findings that had correlated with human cataracts [22]. It has been suggested that a decrease in the antioxidant status of the erythrocytes may increase the oxidative damage in the tissues, which includes the oxidative modification of the lens proteins which are observed in cataracts. However, in contrast to these data, the increased blood levels of the antioxidant enzymes have been reported to be associated with cataracts [22-24].
Conclusion
The oxidative stress of the lens had a direct influence on the solubility of the lens proteins, which led to an increase in the opacity of the lens. The antioxidant enzyme activity levels reflected the changes which took place in the development of senile cataracts. At present, the only remedy is surgical removal of the cataractous lens and substituting it with a lens which is made of synthetic polymers. Therefore, there is a search for an intervention which will help in delaying the onset and in the slowing down of the aetiology of the cataracts. The assays of these enzymes and the plasma TBARS can be used as the biomarkers of the degeneration in the lens.
[1]. Cekic S, Zlatanovic G, Cvetkovic T, Petrovic B, The oxidative stress in cataractogenesisBosian Journal Of Basic Medical Sciences 2010 10(3):265-69. [Google Scholar]
[2]. Mohan M, Sperduto RD, Augra SK, Milton RC, Mathur RL, Underwood BA, An Indo-US case control study on age related cataractsArch Ophthalmol 1989 107:670-76. [Google Scholar]
[3]. Virgolici B, Stoian I, Muscurel C, Maracine M, Popescu L, Moraru C, The systemic redox modifications in senile cataractsRom. J. Intern. Med 2009 47(3):279-87. [Google Scholar]
[4]. Harding JJ, The physiology, biochemistry, pathogenesis and the epidemiology of cataractsCurrent Opinion in Ophthalmology 1992 3:3-12. [Google Scholar]
[5]. Donma O, Yorulniaz E, Pekel H, Suyugul N, The blood and lens lipid peroxidation and the anti-oxidant status in normal individuals and in senile and diabetic cataractous patientsCurr. Eye Res 2002 25(1):9-16. [Google Scholar]
[6]. Yamanaka N, Fukishima M, Koizami K, Nishida K, Kato T, Ota K, Enhancement of the DNA chain breakage by the bleomycin and biological free radicals producing systemOxygen Biomembrane (new York) North Holland 1998 :56-69. [Google Scholar]
[7]. Saadat M, Farvardin Jahroni M, Saadat H., The null genotype of glutathione-S-transferase M1 is associated with the senile cataract susceptibility in non smoker femalesBiochem Biophys. Res Commun 2004 :1287-91. [Google Scholar]
[8]. Satoh K, The serum lipid peroxide levels in cerebrovascular disorders which were determined by a new colorimetric methodClin Chim Acta 1978 90:37-43. [Google Scholar]
[9]. Marklund Marklund, Assaying the superoxide dismutase activity in animal tissuesJ Biochem 1988 13(3):305-15.Modified by Nandi et al [Google Scholar]
[10]. Paglia DE, Valentine WN, Studies on the quantitative and the qualitative characterization of the erythrocyte glutathione peroxidaseJ Lab Clin Med 1967 70:158-69. [Google Scholar]
[11]. David LL, Shearer TR, The role of proteolysis in the lenses: A reviewLens Eye Tox Res 1989 6:725-47. [Google Scholar]
[12]. Chitkara DK, Yanoff M, Duker JS, Cataract formation mechanismsOphthalmology Mosby 2004 4second ed:273-79. [Google Scholar]
[13]. Boulton M, Saxby I.A., Yanoff M, Duker JS, Age changesOphalmology Mosby 2004 4second ed:261-68. [Google Scholar]
[14]. Taylor A, Jacques P, Chylack LT, Haukinson SF, Khu PM, Rogerset G, The long term intake of vitamins and carotenoids and the odds of early age related cortical and posterior sub capsular lens opacities, 1-4Am J Clin. Nutr 2002 75:540-49. [Google Scholar]
[15]. Boscia F, Grattagliano L, Vandermiale G, The protein oxidation and the lens opacity in humansInvest. Ophthalmol.Vis. Sci 2000 41:2461-65. [Google Scholar]
[16]. Babizhayev MA, The accumulation of lipid peroxidation products in human cataractsActa ophthalmol 1989 67:281-87. [Google Scholar]
[17]. Virgolici B, Stoian I, Muscurel C, Maracine M, Moraru C, Dinu V, The plasma redox status in age related cataractsRomanian Journal of Internal Medicine 2009 47(3):279-87. [Google Scholar]
[18]. Xue AN, Cai QY, Wang SQ, Zhou AS, Li Fu P, Chen Biomed Environ Sc 1996 9(2-3):144-48. [Google Scholar]
[19]. Delcourt C, Cristol JP, Tessier F, Leger CL, Mitchel F, Papoz L, The risk factors for cortical, nuclear and posterior subcapsular cataracts: The POLA studyPathologies Occularies Liees Epidemiol 2000 151(5):497-504. [Google Scholar]
[20]. Leutner S, Schindowski K, Frolich L, Muller WE, An enhanced ROS generation in the lymphocytes in Alzheimers patientsPharmacopsychiatry 2005 38(6):312-15. [Google Scholar]
[21]. Meister A, The selective modification of the glutathione metabolismSciences 1983 220(4596):472-77. [Google Scholar]
[22]. Nourmohamadi I, Ladan G, Mehdi M, Abbas GJ, Evaluation of the erythrocyte glutathione peroxidase, superoxide dismutase and the total antioxidant levels in cataract patientsArch Iranian Med 2001 4:123-26. [Google Scholar]
[23]. Delcourt C, Cristol JP, Leger GL, Descomps B, Papoz L, The association of the antioxidant enzymes with cataracts and age related macular degenerationOphthalmology 1999 106:215-22. [Google Scholar]
[24]. Delcourt C, Carriere I, Delage M, Descomps B, Cristol JP, Papoz L, The association of cataracts with antioxidant enzymes and other risk factors: the French age related eye diseases (POLA) prospective studyOphthalmology 2003 110:2318-26. [Google Scholar]