Menstrual cycle, ABR, Estrogen, Progesterone
Introduction
The hormonal changes that occur in a short time span during a menstrual cycle, promote modifications all over a woman’s body, with physical and emotional manifestations being frequently observed. There is a controversy over how the hormonal conditions influence the cerebral electrophysiology. Auditory Evoked Potentials (AEP) may serve as a non-invasive clinical tool in characterizing the electrophysiological phenomena of neural excitation, conduction and transmission across the auditory pathway.
Ovarian hormones have long been known to affect the sensory information processing in the brain. The auditory perception being one of the sensory modalities, it also gets influenced by the sex hormones. Clinical observations strongly suggest that the changes in the gonadal function modify the auditory, olfactory and the taste thresholds [1]. The thresholds for touch, two point discrimination and the perception of light have also been found to vary during the follicular and the luteal phases of the menstrual cycle [2]. Variations in the different hormonal levels, especially in the levels of oestrogen and progesterone across the menstrual cycle, have been proposed to be responsible for the latency changes of the waves of the auditory evoked potentials [3].
Several researchers have reported that the changes in the auditory acuity occur during the menstrual cycle. In an attempt to specify the critical functional components which are involved in the menstrual fluctuations, the evidence of a better performance in a variety of tasks at mid-cycle, was obtained in a study. It was also shown that some tasks were particularly susceptible to alterations due to the biochemical changes of the menstrual cycle [4]. Ovarian hormones have been found to modulate the conduction of sensory information, by many researchers. Numerous tasks which include the visual and the auditory thresholds vary systematically throughout the menstrual cycle. A reduction in the threshold during menstruation implied that the withdrawal of the sex hormones improved the hearing [5,6].
The influence of oestrogen and other gonadal steroid substances may have direct effects on the cochlea and on various central auditory system pathways. This could indirectly influence the central processing through other pathways and it could also modulate the blood flow in the cochlea and the brain [7]. In one study, it was suggested that oestrogen might influence the acetylcholine synthesis, which was recently shown to be present in the auditory system [8]. The sex steroids have been shown to interact directly with the surface membrane receptors to change the excitability of the nerve cells in the hypothalamus and the hippocampus [9,10].
Homeostasis and the biochemical status of the inner ear fluid are essential for hearing. The changes in sodium and the water reabsorption that take place during the menstrual cycle, may affect the functioning of this part of the peripheral auditory system, and this may affect the homeostasis, which may cause auditory and labyrinthic symptoms [11].
Different results have been reported by various authors in different studies regarding the impact of the physiological fluctuations in the female hormones on the auditory evoked responses, as was assessed in different phases of the menstrual cycle. The aim of the present study was to evaluate the correlation of different parameters of the Brainstem Auditory Evoked Responses with the female sex hormones.
Material and Methods
This study was conducted in the Department of Physiology, Government Medical College, Amritsar, India, over a one year period, after a clearance from the institutional “Ethics Committee” was obtained. The participants were informed about the study, that the testing procedures were quite simple, non-invasive and harmless for the subjects. They were then asked to sign a free and informed consent form before they were included in this study.
This study was conducted on 50 female subjects (undergraduate and post graduate students) who were in the age group of 19-36 years. All of them had regular menstrual cycles of 28-30 days and they had not taken any hormonal pills during the past 6 months. Only those subjects who had a Body Mass Index (BMI) which was within the normal range (18.5-24.99 kg/m2) were included in the study. Any subject who reported any dysendocrinism or metabolic or neoplastic pathologies was excluded from this study. Tuning fork tests were performed to exclude any conduction defect. A detailed menstrual history was taken from these subjects and a general physical examination was also done.
The subjects who were included in this study were then familiarized with the procedure and the nature of the test, in order to allay any remaining fear or apprehension.
During their menstrual cycles, each woman underwent four ABR tests, the first at the menstrual phase (day 1-3), the second at the mid-cycle (day 11-14), the third at the mid-luteal phase (day 17-22) and the fourth at the pre-menstrual phase (day 25-27). A retrospective calculation of the approximate day of ovulation was done from the day of onset of the next menstrual cycle. If any discrepancy was found, then that cycle was excluded from the study.
The recordings were obtained by using the RMS EMG EP MARK II version: 7.5.7 2Ch (PC-based) machine in an electrically and acoustically shielded air conditioned room. The recordings of all the subjects were timed at around the same time of the day. During the recording session, the subjects were asked to lie down in the supine position on a reclined bed. The recordings were taken by placing 4 Ag/AgCl disc electrodes on the scalp. The sites of application of the electrodes were: the reference electrode at the vertex (Cz) and the active electrodes at the left and right ear lobes (A1, A2). The grounding was done by placing an electrode on the forehead (Fpz). All the electrodes were placed after cleaning the sites with spirit swabs and they were then affixed with an electrode paste. All the electrodes were plugged to a junction box. The contact impedance was constantly monitored with an impedance meter and the skin to electrode impedance was kept below 5kohms.
The signals which were picked up by these electrodes from the scalp after the standard click stimuli were delivered through the headphones were filtered, amplified, averaged and displayed on the screen of the PC which was attached to the machine. The machine was provided with an inbuilt automatic artifact rejection facility.
For recording the ABR, first, the normal threshold of both the ears of every subject was recorded and then, 2000 click stimuli which had an intensity of 60dB above the normal hearing threshold were given to each ear independently, at the rate of 11.1/sec and for a duration of 0.1 ms. The stimulation was of the rare-type, with a linear envelope. The sweep speed was 1ms/division and the sensitivity was 0.5µV/div. These clicks were generated by passing 0.1 ms square pulses through shielded headphones. After filtration (at 100Hz and 3 KHz), the amplification and averaging of the waves in the first 10 ms of the latency were considered for ABR.
The peak latencies of the waves, I, II, III, IV and V, the inter peak latencies of I-V, I-III and III-V, the amplitudes of waves I and V and the amplitude ratio (V/I) were recorded by using a visual overlay cursor which represented 3-4 min of the data collection.
Both the ears of each woman were treated as independent samples and thus, the ABR wave forms were analyzed separately for each ear. The ears were considered as independent samples because the pathways from the two ears were largely separate anatomically and they were capable of presenting different wave forms in the same individual. The average of the latencies which were extracted from the ABR waveforms which were collected from both the ears was calculated for each woman, because the differences were negligible. A paired data t-test was used to compare each phase of the menstrual cycle. All the recorded values were reported as mean ± SD. A p-value of <0.05 was considered to be statistically significant.
Results
The absolute latencies of the waves, I-V [Table/Fig-1 & 2] showed:
A statistically significant increase in the proliferative phase (phase 2) subjects as compared to that in the menstrual phase (phase1) subjects.
A highly significant to a significant decrease in the secretory phase (phase 3) as compared to that in the proliferative phase (phase 2).
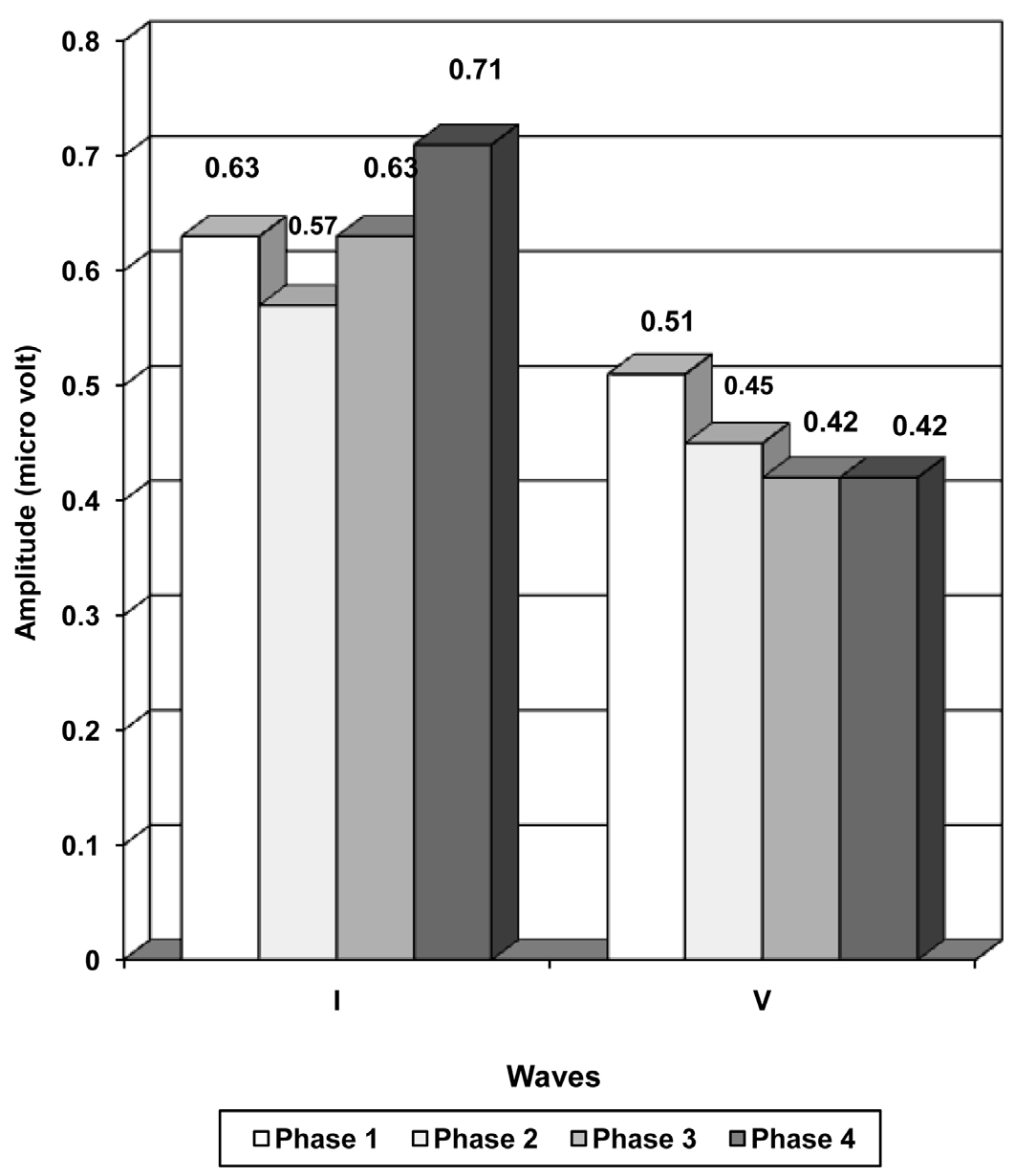
The inter peak latencies, amplitudes and the amplitude ratio depicted a trend of increase in phase 2, a decrease in phase 3 and an increase in phase 4, but they did not show any significant difference in between the phases [Table/Fig-3 and 4].
Discussion
The absolute latencies of the waves, I to V showed a statistically significant increase in the phase 2 subjects as compared to the phase 1 subjects [Table/Fig-2]. The data revealed highly significant (p<0.001) to significant (p<0.05) variations (decrease) in the absolute latencies of the waves, I to V in the phase 3 (secretory) subjects as compared to those in the proliferative phase (phase 2) subjects. The statistically insignificant variations (p>0.05) in IPL I-III, I-V and III-V in both the cases which have been discussed above, can be explained to be due to an overall prolongation of the absolute latencies of the waves, I, III and V.
Latencies and amplitudes of different waves of brain stem auditory evoked potentials in different phases of menstrual cycle.
| Phase 1 | Phase 2 | Phase 3 | Phase 4 |
|---|
| Latency(ms) Mean±SD |
| I | 1.54 + 0.17 | 1.60 + 0.18 | 1.55 + 0.20 | 1.57 + 0.19 |
| II | 2.57 + 0.31 | 2.90 + 0.14 | 2.61 + 0.20 | 2.65 + 0.18 |
| III | 3.50 + 0.15 | 3.60 + 0.17 | 3.53 + 0.17 | 3.54 + 0.16 |
| IV | 4.65 + 0.19 | 4.78 + 0.18 | 4.69 + 0.15 | 4.70 + 0.18 |
| V | 5.52 + 0.23 | 5.62 + 0.22 | 5.52 + 0.25 | 5.56 + 0.20 |
| Inter-peak Latency(ms) Mean ±SD |
| I-III | 1.96 + 0.25 | 2.00 + 0.23 | 1.98 + 0.19 | 1.97 + 0.24 |
| I-V | 3.97 + 0.27 | 4.02 + 0.28 | 3.97 + 0.31 | 3.99 + 0.26 |
| III-V | 2.02 + 0.25 | 2.02 + 0.28 | 2.00 + 0.28 | 2.02 + 0.22 |
| Amplitudes (|liv) Mean± SD |
| I | 0.63 + 0.24 | 0.57 + 0.13 | 0.63 + 0.25 | 0.71 + 0.34 |
| V | 0.51 + 0.20 | 0.45 + 0.14 | 0.42 + 0.19 | 0.42 + 0.22 |
| Amplitude ratio (V/I) Mean ± SD |
| AR | 1.15 + 0.93 | 0.86 + 0.36 | 0.77 + 0.31 | 0.89 + 1.05 |
Figure showing the wave latencies in different phases of menstrual cycle
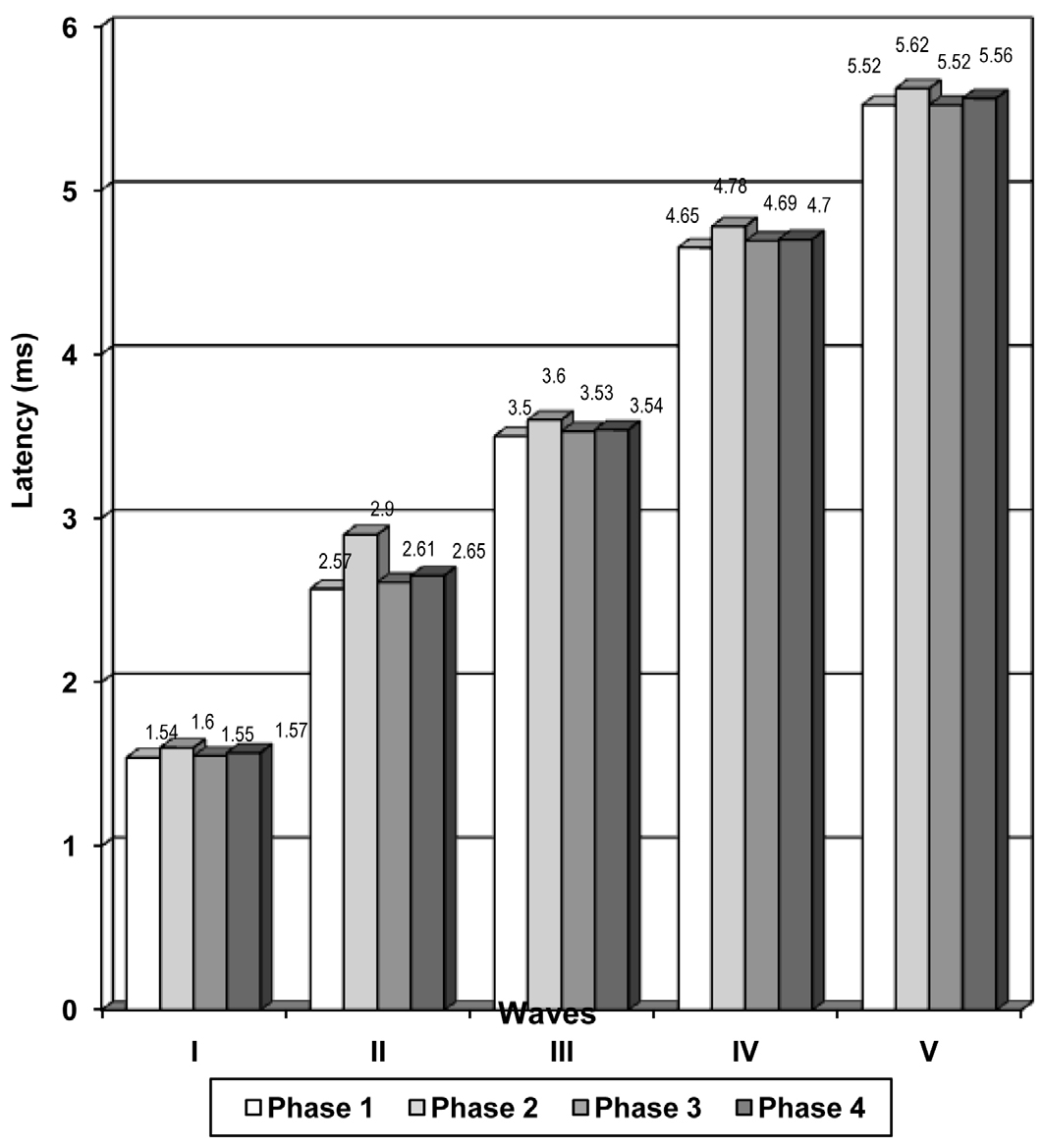
Figure showing the inter-peak latencies in different phases of menstrual cycle
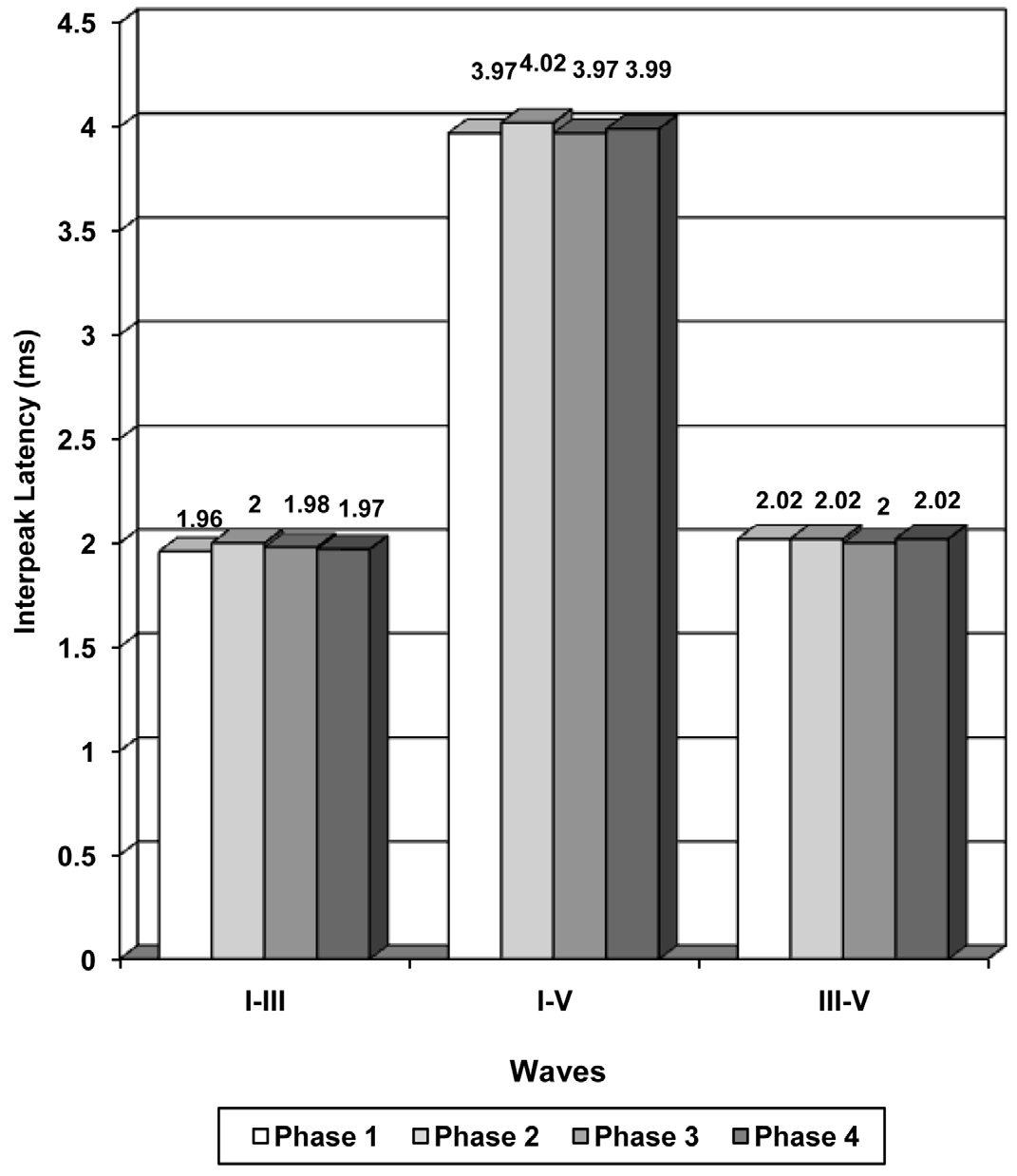
Figure showing the amplitudes of waves in different phases of menstrual cycle
In the present study, there was a statistically significant increase in the peak latencies of the waves, I, II, III, IV and V during the oestrogen peak mid-cycle phase and a decrease in the latencies which occurred during the progesterone peak mid-luteal phase. This showed that the ovarian steroids, oestrogen and progesterone, affected the synaptic transmission at the level of the brainstem. The probable explanation is the modulation in the secretion of GABA in a counter-regulatory fashion. Oestrogen may enhance the inhibitory effects of GABA by stimulating its secretion, thereby delaying its conduction. Conversely, progesterone may decrease the sensitivity of the neurons and blunt the oestrogen potentiated GABA release [12].
Oestrogen has been shown to have a dual action on the GABA release in female primates. A study demonstrated that the pulsatile release of the hypothalamic GABA was significantly increased in female monkeys at the time of an oestrogen surge and that it was abolished during the early follicular and the late luteal phases [13]. This biphasic response of GABA to the oestrogen action helps in further explaining the rise in the latencies during the mid-cycle in ovulating females. At mid-cycle, oestrogen has a positive feedback action which results in an enhanced GABA secretion in the brain, whereas it has a negative feedback action during the early follicular and the late luteal phases.
In the present study, the peak latencies of all the waves (I to V) further increased during the pre-menstrual phase. This suggested that the neuronal conduction process at the auditory pathway was relatively slower pre-menstrually. The exact mechanism which is responsible for such a change is not known. The retention of water and sodium due to the highest levels of both oestrogen and progesterone could be one of the mechanisms which influence the process of the axonal conduction time [14]. The other mechanisms could be progesterone withdrawal during the late luteal phase and an increased secretion of prolactin, aldosterone and the anti diuretic hormones. Beta-endorphin withdrawal and the direct sedative effect of the prostaglandins on the central nervous system could cause a delayed conduction [15].
Summary and Conclusions
From the present study, it can be concluded that the absolute latencies of the various BAEP waves in the proliferative phase were increased, which showed a slower neural conduction. This can be attributed to the high levels of oestrogen during the proliferative phase of the menstrual cycle. On the other hand, the absolute latencies of all the above waves of the BAEP parameters showed a decrease in the secretory phase and hence, this enhanced the conduction across the neural pathways. This can be due to the thermogenic effect of the hormone, progesterone, as well as its antagonistic effect to oestrogen.
The study needs to be further elaborated regarding the estimation of the blood levels of oestrogen and progesterone in all the phases.
[1]. Vogel W, Broverman DM, Klaiber EL, EEG responses in regularly menstruating women and in amennorrheic women treated with ovarian hormonesScience 1971 172:388-91. [Google Scholar]
[2]. Yadav A, Tandon OP, Vaney N, Auditory evoked responses during different phases of menstrual cycleIndian J Physiol Pharmacol 2002 46(4):449-56.Henkin RI, Editor. Sensory changes during the menstrual cycle. In: Biorythm and human reproduction. New York: John-Wiley and sons. p. 277. [Google Scholar]
[3]. Diamond M, Diamond AL, Mast M, Visual sensitivity and sexual arousal levels during the menstrual cycleJ Nerve Ment Dis 1972 152:170-76. [Google Scholar]
[4]. Haggard M, Gaston JB, Changes in auditory perception in the menstrual cycleBr J Audiol 1978 12(4):105-18. [Google Scholar]
[5]. Fagan PL, Church GT, Effect of menstrual cycle on the auditory brain stem responseAudiology 1986 25(6):321-28. [Google Scholar]
[6]. Howard R, Mason P, Taghavi E, Spears G, Brainstem auditory evoked responses during the menstrual cycle in women with and without premenstrual syndromeBiol Psych 1992 32:682-90. [Google Scholar]
[7]. Caruso S, Maiolino L, Rugolo S, Intelisano G, Farina M, Cocuzza S, Auditory brain stem response in premenopausal women taking oral contraceptivesHum Reprod 2003 18(1):85-89. [Google Scholar]
[8]. Picton TW, Hillyard SA, Krausz HI, Galambos R, Human Auditory evoked potentials-evaluation of componentsElectro Encephalogr ClinNeurophysiol 1974 36:179-90. [Google Scholar]
[9]. McEwen BS, Davis PG, Parsons B, Pfaff DW, The brain as target for steroid hormone actionAnn Rev Neurosci 1979 2:65-73. [Google Scholar]
[10]. Curtis DR, Game CJA, Johnston GAR, McCulloch RM, Central effects of β-(p chlorophenyl)-γ-aminobutyricBrain Res 1974 70:493-99. [Google Scholar]
[11]. Arruda PO, Silva IMC, Study of oto-acoustic emissions during the female hormonal cycleRev Bras Otorhinolaryngol 2008 74(1):106-11. [Google Scholar]
[12]. Martin MR, Baclofen and the brain stem auditory evoked potentialsExperimental Neurology 1982 76:675-80. [Google Scholar]
[13]. Roosen-Runge G, Epler M, Duker E, Siegel RA, Demling J, Wuttka W, In vivo release of neurotransmitters in the medial basal hypothalamus of the monkeyExp Brain Res 1984 54(3):575-78. [Google Scholar]
[14]. Bruce J, Russell GFM, Premenstrual tension – a study of weight changes and balances of sodium, water and potassiumLancet 1962 II:267-71. [Google Scholar]
[15]. Wehrenberg WB, Wardlow SL, Frantz AG, Ferin M, Beta-endorphin in hypophyseal portal blood-variations throughout the menstrual cycleEndocrinology 1982 111(3):879-81. [Google Scholar]