INTRODUCTION
Oral squamous cell carcinoma remains a serious problem of oral health worldwide. Globally, head and neck squamous cell carcinoma is the sixth most common malignancy and it accounts for approximately 5% of the malignant tumours in the developed countries. Whereas, in the developing countries, it is the most common malignancy which accounts for up to 50% of the malignant tumours.
Oral squamous cell carcinoma has a complex biological behaviour and despite the advances in the treatment modalities, the 5-year survival rates of the patients with oral squamous cell carcinoma have improved only slightly. This has led to an interest in predicting its possible future behaviour, so that alternative therapeutic strategies can be tailored to treat the severity of the tumour.
The molecular biological markers of OSCC have been extensively studied to aid in the prevention and the prognosis of OSCC. However, no marker has been universally accepted so far.
Angiogenesis or neovascularization, which is the formation of a new microvasculature, is an important component in many biological processes, both in physiological conditions, such as the proliferating endometrium and embryogenesis, and in pathological conditions, such as rheumatoid arthritis and neoplastic diseases [1]. Angiogenesis has been known to aid in the progression and the metastasis of many malignant tumours which include tumours of the lung [2], breast [3], oesophagus [4,5] and the oral cavity [6–12].The induction of angiogenesis is mediated by several stimulatory and inhibitory molecules which are released by both the tumour and the host cells and it depends on a net balance between the stimulatory angiogenic and the inhibitory anti-angiogenic factors [13].
Among the various host immune cells, the mast cells have been implicated in tumour progression because they promote angiogenesis [5,14]. To examine the relationship between the mast cell density and the histological grade of OSCC, we analyzed the mast cell density (MCD) in different grades of OSCC and compared it with that of the normal mucosa by using the 1% Toluidine blue stain as the mast cells stain metachromatically with Toluidine blue.
SUBJECTS AND METHODS
Formalin-fixed, paraffin-embedded tissue specimens of 102 cases of OSCC (40 well differentiated OSCC, 50 moderately differentiated OSCC, and 12 poorly differentiated OSCC) were retrieved from the archives.
Seven cases of normal tissues were included in the study as controls. The relevant information regarding the clinical parameters was obtained from the records of the patients.
The cases which were diagnosed clinically and confirmed by histopathological means as squamous cell carcinoma alone, were included in the study. These cases were graded histologically into well differentiated, moderately differentiated and poorly differentiated squamous cell carcinomas.
In brief, 5 μm sections of formalin-fixed, paraffin blocks were deparaffinized with xylene and they were rehydrated with graded alcohols. The slides were stained with 1% Toluidine blue, mounted with DPX and observed under a microscope. The stained sections were studied for metachromasia, which was taken as a positive identification of the mast cells.
Sections of neurofibromas were used as the positive controls for the mast cells.
The slides were studied under a light microscope. The mast cell granules stained brilliant red/purple and the background stained in different shades of blue.
For the determination of the Mast Cell Density (MSD), the stained sections were screened at low power (160X) to identify the areas of the hot spots. A mast cell count was performed at the 640X magnification in three randomly chosen fields in the hot spot areas. The mast cell count was expressed as the number of mast cells per high power field. The average figures which were obtained in the counted hot spot fields were considered as the MCD for a given case. All the counts were performed by a single investigator who had the knowledge of the clinical or the histopathological variables, to eliminate an interobserver variation.
The MCD between OSCC and the normal oral mucosa and among the various grades of OSCC were compared by using the independent Student’s ‘t’ test and ANOVA. The statistical correlation of MCD between the various grades in OSCC was analyzed by using Pearson’s correlation coefficient.
RESULTS
A retrospective study was performed and the study sample comprised of 102 cases of oral squamous cell carcinomas, which consisted of 40 cases of well-differentiated squamous cell carcinomas, 50 cases of moderately-differentiated squamous cell carcinomas, and 12 cases of poorly- differentiated squamous cell carcinomas. 07 cases of normal tissues were included in the study as controls [Table/Fig-1].
Histopathological grading of OSCC in the study group and Normal tissue
| Histopathological Diagnosis | No. of Cases |
| Normal | 07 |
| Well differentiated squamous cell carcinoma | 40 |
| Moderately differentiated squamous cell carcinoma | 50 |
| Poorly differentiated squamous cell carcinoma | 12 |
| Total | 109 |
By using the independent ‘t’-test, the MCD was found to be significantly lower in WDSCC (6.22±0.66/HPF) than in the normal oral mucosa (11.42±1.21/HPF); the MCD was found to be lower in MDOSCC (4.64±0.58/HPF) as compared to WDOSCC (6.22± 0.66/HPF) and the normal oral mucosa (11.42±1.21/HPF). Similarly, the MCD was found to be significantly lower in PDOSCC (1.06±0.39/HPF) as compared to the normal oral mucosa (11.42 ± 1.21/HPF) and WDSCC (6.22±0.66/HPF) (P=> 0.01 which was significant at a 1% level) [Table/Fig-2]
Average number of mast cells (per/HPF-640x) in different histological grades of OSCC and normal tissue in the study group.
| Histopathological Diagnosis | Mast Cell Density (mSD) (mean±Sd) |
| NORMAL | 11.42±1.21 |
| WDSCC | 6.22± 0.66 |
| MDSCC | 4.64± 0.58 |
| PDSCC | 1.06± 0.39 |
WDSCC – Well differentiated Squamous cell carcinoma
MDSCC – Moderately differentiated squamous cell carcinoma
PDSCC – Poorly differentiated squamous cell carcinoma
As shown in [Table/Fig-3A] the Spearman’s rank correlation coefficient revealed a significant correlation between the density of the mast cells and the increasing grade of OSCC [Table/Fig-3 and 4].
Comparison of average number of mast cells between different grades of oral squamous cell carcinoma
p < 0.01 – Significant at 1% level
| WDSCC | MDSCC | PDSCC | F-Value | p-Value |
| (N = 40) | (N = 50) | (N = 12) |
| Mean | + | SD | Mean | + | SD | Mean | + | SD |
| 6.22 | + | 0.66 | 4.64 | + | 0.58 | 1.06 | + | 0.39 | 8.12 | < 0.01 |
Comparison of Average Number of Mast Cells in Different Grades of SCC
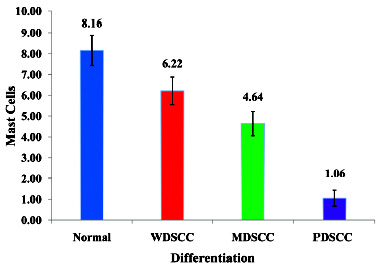
Comparison of average number of mast cells between any two grades of oral squamous cell carcinoma
p > 0.05 – Not significant; p < 0.01 – Significant at 1% level
| WDSCC | MDSCC | PDSCC | F-Value | p-Value |
| (N = 40) | (N = 50) | (N = 12) |
| Mean | + | SD | Mean | + | SD | Mean | + | SD |
| 6.22 | + | 0.66 | 4.64 | + | 0.58 | | | | 1.80 | > 0.05 |
| 6.22 | + | 0.66 | | | | 1.06 | + | 0.39 | 4.17 | < 0.01 |
| | | 4.64 | + | 0.58 | 1.06 | + | 0.39 | 2.99 | < 0.01 |
DISCUSSION
A sustained tumour growth requires a positive balance between the tumour cell proliferation and cell death or apoptosis. By using an experimental animal model, it was shown that the initiation of angiogenesis appeared concomitantly with a decrease in the tumour cell apoptosis, while the levels of the tumour cell proliferation remained constant, thus leading to the net tumour growth [10]. The preinvasive malignant cells are known to remain dormant until they become angiogenic, and this is followed by a phase of rapid tumour growth [15,16].
Angiogenesis is the outcome of an imbalance between the positive and the negative angiogenic factors which are produced by both the tumour and the host cells [13]. Among the host cells which produce and release the pro-angiogenic and the angiogenic factors are the mast cells [13]. The mast cells are an important source of several proangiogenic and angiogenic factors such as histamine, heparin, chymase, the basic Fibroblast Growth Factor (bFGF), the vascular endothelial growth factor (VEGF), the transforming growth factor-beta (TGF-b) and others [13].
The tumour angiogenesis and tumour growth have been reported to be less in mast cell deficient mice as compared to those in the mice with normal mast cell numbers [17]. Moreover, the mast cells were shown to induce neovascularization through the carcinogenesis of the squamous cells [18]. The angiogenic factors which include VEGF, bFGF, and the platelet-derived growth factors have been reported to stimulate the mast cell migration. Nineteen Hypoxia might induce the tumour cells to release angiogenic factors, which in turn could chemo attract the mast cells to migrate into the hypoxic areas of the tumour. After their migration into the hypoxic areas, the mast cells may produce angiogenic products that may stimulate the infiltration of even more mast cells [19].
Various angiogenic factors which are secreted by the mast cells either directly promote angiogenesis by stimulating the migration and/or proliferation of the mast cells or indirectly through the degradation of the extracellular matrix [19].
The density of the mast cells in a tissue was studied by using histochemical stains like toluidine blue and [5] alcian blue [2] and immunohistochemically [20] by using mast cell tryptase, heparin, chymase, and carboxypeptidase A. The present study employed the quantification of the mast cells in the area of the ‘hot spots’. The mast cells were stained with a metachromatic dye, 1% aqueous Toluidine blue and the areas which represented the highest number of mast cells were found by scanning the tumour sections under the 160X magnification. After the identification of the ‘hot spots’, the individual mast cell counts were made under the 640 X magnification in 3 randomly chosen high power fields (HPFs). The average count of 3 HPFs (640X) was considered as the final count for that case.
In the present study, the average number of the mast cells/ HPF was significantly more for the well-differentiated (6.21±0.66) and the moderately-differentiated (4.58±0.58) oral squamous cell carcinomas as compared to that for the poorly-differentiated (1.05±0.39) oral squamous cell carcinomas (p<0.01).
These findings were in stark contrast to those which were reported in previous studies which were done on various tumours [21-25].
However, Oleiveira-Neto HH et al. found the MCD to be lower in OSCC and in premalignant lesions as compared to that in the normal controls [22]. They attributed it to the migration failure of the mast cells, which possibly reflected a modification in the microenvironment during the tumour initiation and progression. Certain researchers have shown the antitumour functions of the mast cells, which include natural cytotoxicity and the release of antitumour compounds [2].
Tomita M et al., [2] have put forth two reasons for such conflicting reports on the role of the mast cells. The cytotoxic functions of the mast cells that suppress the tumour activities may be present initially when the mast cells infiltrate the tumour tissue. However, after the infiltration, the tumour cells may promote the angiogenic properties of the mast cells while suppressing their cytotoxic functions, thereby leading to tumour angiogenesis [2]. Secondly, the cell-mediated cytotoxic effects of the mast cells have been reported, with mast cell: tumor ratios which were greater than 20:1 [2]. Conversely, the cytotoxic effects of the mast cells were nullified and the tumour progression was found to be enhanced when the mast cell-tumor ratios were increased from 10:1 to 1:100 [2]. Hence, the effect of the mast cells against the cancer cells might depend on the concentration of the mast cell products in the microenvironment. Based on these findings, Tomita M et al. [2] hypothesized that reversing this process, i.e., enhancing the cytotoxic functions of the mast cells and suppressing their angiogenic functions, could lead to a new anti-cancer treatment strategy. Furthermore, the mast cell heparin inhibitors, protamine and platelet factor 4, have been reported to inhibit angiogenesis [2].
In the present study, the correlation between MCD and the progression of oral squamous cell carcinoma from well differentiated to poorly differentiated revealed a linear decrease in the MCD, thus suggesting a negative correlation between them. However, if the presence of the mast cells was the key factor in the angiogenesis, there would have been an exponential increase rather than a decrease, thus indirectly suggesting the role of other factors that could have modulated the angiogenesis.
Our results were in concordance with those of Coussens LM et al., [26] study on role of the mast cells during squamous epithelial carcinogenesis in a mouse model. They observed that the stroma in poorly-differentiated carcinoma was devoid of mast cells. They suggested that the angiogenic regulation in squamous carcinogenesis was biphasic. In the early phases, angiogenic activators are released via the mast cell degranulation and as the neoplastic progression proceeds, the angiogenic growth factor gene expression is upregulated in the cancer cells, wherein the tumour cells control the angiogenic phenotype directly, instead of depending upon the manipulation of the inflammatory cells to indirectly affect the neovascularization.
The progressive depletion of the mast cells in the present study from well and moderately differentiated OSCC to poorly differentiated OSCC, could be probably because the mast cells may have been degranulated as the disease progressed. The lack of mast cell granules in the advanced disease states may have resulted in the negative staining with Toluidine blue. This was substantiated by the ultra structural observation of the mast cells in different stages of degranulation and their progressive reduction and disappearance in the advanced states by Rajendran R et al. [27] in OSMF and by Clamon N H et al., in GVHD [28].
Sudhakar R et al. [29] have also shown an inverse relationship between the mast cells and their vascularity and inflammation, in oral inflammatory lesions.
Finally, the lack of a consensus between the mast cell and the micro vascular densities in OSCC in different studies can be explained as follows:
The growth of a solid tumour is dependent upon an adequate blood supply, which is achieved by the generation of stroma, where the formation of capillaries is a central event and it is also an entry site for the immune inflammatory cells. In other words, in a particular tumour, the number of the micro vessels and the mast cells could be related to the amount of the stromal component. Consequently, the tumours which have more stroma may have more micro vessels and mast cells. For this reason, the variations in the amount of stroma and the tumour cells may influence the average number of the mast cells [23].
These discordant results could also be due to different methodologies in the assessment of the MCD and the interobserver variation. Although these factors posed potential limitations for a comparison between studies, our results suggested that MCD was useful for detecting the aggressive behaviour in the tumour cells in oral squamous cell carcinoma.
Our study demonstrated a significant statistical correlation between the densities of the mast cells in the advancing grades of oral squamous cell carcinoma (p<0.01, significant at a 1% level).
The significantly higher densities of the mast cells in well and moderately-differentiated OSCC as compared to those in poorlydifferentiated OSCC strongly suggest that the mast cell density may be used as an indicator for the disease progression in the oral carcinogenesis. This finding has a clinical significance in helping in delineating a risk population, which might benefit from adjuvant therapeutic strategies e.g. the mast cell degranulation blocking therapy, But it remains to be tested whether the inhibition of the tumour angiogenesis is feasible when the tumour vessels are mosaically lined by the tumour cells and when the tumours easily find escape routes to switch on to alternative angiogenic programs.
CONCLUSION
The decrease in the mast cell numbers might possibly reflect an important modification in the microenvironment during the tumour initiation and progression. Currently, the exact functional relevance of the mast cells which surround various tumours is unclear. However, the accumulated evidence indicates that the mast cells may induce the tumour progression by providing a mitogenic stimulation or angiogenesis - the hallmark of the tumour growth and metastasis through the release of various mediators.
MCD may be used as an indicator of the disease progression by helping in delineating a risk population, which might benefit from an attractive adjuvant therapeutic strategy for OSCC. The role of the mast cells in the angiogenesis in OSCC and the role of angiogenesis in the tumour progression need to be further validated by using larger samples that include recurrent cases and follow up studies.
WDSCC – Well differentiated Squamous cell carcinomaMDSCC – Moderately differentiated squamous cell carcinomaPDSCC – Poorly differentiated squamous cell carcinoma
[1]. Tae K, El-Naggar AK, Yoo E, Feng L, Lee J, Hong WK, Expression of the vascular endothelial growth factor and the micro vessel density in the head and neck tumorigenesisClin Cancer Res. 2000 6:2821-28. [Google Scholar]
[2]. Tomita M, Matsuzaki Y, Onitsuka T, The effect of mast cells on the tumor angiogenesis in lung cancerAnn Thorac Surg. 2000 69:1686-90. [Google Scholar]
[3]. Weidner N, Semple JP, Welch WR, Folkman J, Tumor angiogenesis and metastasis – correlation in invasive breast carcinomaN Engl J Med. 1991 324:1-8. [Google Scholar]
[4]. Igarashi M, Dhar DK, Kubota H, Yamamoto A, EL-Assal O, Nagsue N, The prognostic significance of the micro vessel density and thymidine phosphorylase expression in squamous cell carcinoma of the esophagusCancer 1998 82:1225-32. [Google Scholar]
[5]. Elpek GO, Gelen T, Akshoy NH, Erdogan A, Dertsiz L, Demircan A, The prognostic relevance of angiogenesis and the mast cells in squamous cell carcinoma of the esophagusJ Clin Pathol. 2001 54:940-44. [Google Scholar]
[6]. Williams JK, Carlson GW, Cohen C, Derose PB, Hunter S, Jurkiewicz MJ, Tumor angiogenesis as a prognostic factor in oral cavity tumorsAm J Surg. 1994 168:373-80. [Google Scholar]
[7]. Jin Y, Tipoe GL, White FH, Yang L, A quantitative investigation of the immunocytochemically stained blood vessels in normal, benign, premalignant and malignant human oral cheek epitheliumVirchow’s Arch. 1995 427:145-51. [Google Scholar]
[8]. Tipoe GL, Jin Y, White FH, The relationship between the vascularity and the cell proliferation in human normal and pathological lesions of the oral cheek epitheliumOral Oncol. 1996 32:24-31. [Google Scholar]
[9]. Carlie J, Harada K, Baillie R, Macluskey M, Chisholm DM, Ogden GR, Vascular endothelial growth factor (VEGF) expression in the oral tissues: the possible relevance in angiogenesis, tumor progression and field cancerisationJ Oral Pathol Med. 2001 30:449-57. [Google Scholar]
[10]. Macluskey M, Chandrachud LM, Pazouki S, Green M, Chisholm DM, Ogden GR, Apoptosis, proliferation, and angiogenesis in the oral tissues: the possible relevance in the tumour progressionJ Pathol. 2000 191:368-75. [Google Scholar]
[11]. Ravi D, Ramadas K, Mathew BS, Nalinakumari KR, Mair MK, Pillai MR, The angiogenesis during tumor progression in the oral cavity is related to a reduced apoptosis and a high tumor cell proliferationOral Oncol. 1998 34:543-48. [Google Scholar]
[12]. Iamaroon A, Pongsiriwet S, Jittidecharaks S, Pattanaporn K, Prapayasatok S, Wanachantararak S, The increase in the number of mast cells and the tumor angiogenesis in oral squamous cell carcinomaJ Oral Pathol Med. 2003 32:195-99. [Google Scholar]
[13]. Michailidou EZ, Markopoulos AK, Antoniades DZ, Mast cells and angiogenesis in oral malignant and premalignant lesionsThe Open Dent J. 2008 2:126-32. [Google Scholar]
[14]. Tomita M, Matsuzaki Y, Edagawa M, Shimizu T, Hara M, Sekiya R, Association of the mast cells with the tumor angiogenesis in esophageal squamous cell carcinomaDis Esophagus. 2001 14:135-38. [Google Scholar]
[15]. Shieh YS, Lee HS, Shiah SG, Chu YW, Wu CW, Chang LC, The role of angiogenic and non-angiogenic mechanisms in oral squamous cell carcinoma: correlation with the histologic differentiation and the tumor progressionJ Oral Pathol Med. 2004 33:601-06. [Google Scholar]
[16]. Moriyama M, Kumagai S, Kawashiri S, Kojima K, Kakihara K, Yamamoto E, An immunohistochemical study on the tumor angiogenesis in oral squamous cell carcinomaOral Oncol. 1997 33:369-74. [Google Scholar]
[17]. Dethlefsen SM, Matsuura N, Zetter BR, Mast cell accumulation at sites of murine tumour implantation: the implications for angiogenesis and tumour metastasisInvasion Metastasis 1994/5 14:395-408. [Google Scholar]
[18]. Coussens LM, Raymond WW, Bergers G, Laig-Webster M, Behrendtsen O, Werb Z, The inflammatory mast cells up-regulate the angiogenesis during squamous epithelial carcinogenesisGenes Dev. 1999 13:1382-97. [Google Scholar]
[19]. Gruber BL, Marchese MJ, Kew R, Angiogenic factors stimulate the mast-cell migrationBlood 1995 86:2488-93.Available from: http://www.antibodybeyond.com [Google Scholar]
[20]. Iamaroon A, Pongsiriwet S, Jittidecharaks S, Pattanaporn K, Prapayasatok S, Wanachantararak S, The increase in the number of mast cells and the tumor angiogenesis in oral squamous cell carcinomaJ Oral Pathol Med. 2003 32:195-99. [Google Scholar]
[21]. Michailidou EZ, Markopoulos AK, Antoniades DZ, The mast cells and angiogenesis in oral malignant and premalignant lesionsThe Open Dentistry Journal 2008 2:126-32. [Google Scholar]
[22]. Elpek GO, Gelen T, Aksoy NH, Erdogan A, Dertsiz L, Demircan A, The prognostic relevance of angiogenesis and the mast cells in squamous cell carcinoma of the oesophagusJ Clin Pathol. 2001 54:940-44. [Google Scholar]
[23]. Tomita M, Matsuzaki Y, Onitsuka T, The effect of mast cells on the tumor angiogenesis in lung cancerAnn Thorac Surg. 2000 69:1686-90. [Google Scholar]
[24]. Bhushan S, Sriram G, Saraswathi TR, Sivapathasundharam B, The immunohistochemical evaluation of the mast cells and the angiogenesis in oral squamous cell carcinomaIJDR 2010 21:260-65. [Google Scholar]
[25]. Yong L.C., The mast cell: origin, morphology, distribution and functionExp Toxicol Pathol. 1977 49(6):409-24.(Abstract Obtained from Medline) [Google Scholar]
[26]. Coussens LM, Raymond WW, Bergers G, Laig-Webster M, Behrendtsen O, Werb Z, Inflammatory mast cells up-regulate the angiogenesis during squamous epithelial carcinogenesisGenes and Development. 1999 13:1382-97. [Google Scholar]
[27]. Rajenderan R, Radhakrishnan NS, Kartha C, Light and electron microscopic studies on oral sub mucous fibrosisJ Indian Dent Assoc. 1993 64:157-61. [Google Scholar]
[28]. Claman NH, Choi KL, Walter S, Watter AE, Mast cell disappearance in chronic murine GVHD- the ultra structural demonstration of the phantom mast cellsJ Immunol. 1986 137:2009-13. [Google Scholar]
[29]. Sudhakar R, Ramesh V, Balamurali PD, Nirima Oza, Premalatha B, Karthikshree V, The incidence of mast cells in oral inflammatory lesionsJ Oral and Maxillofacial Pathology Jan-Jun2005 :9 [Google Scholar]