Introduction
An Adverse Drug Reaction (ADR) is a response to a drug which is noxious and unintended and which occurs at doses which are normally used in man for the prophylaxis, diagnosis or the therapy of a disease and for the modification of its functions, which excludes its failure to accomplish the intended purpose [1]. ADRs have been implicated as a leading cause of considerable morbidity and mortality. The incidence of ADRs varies with studies, which show incidences which range from as low as 0.15% to as high as 30% [2]. They are a major clinical problem, accounting for 2-6% of all the hospital admissions [3].
India rates below 1% in terms of ADR reporting against the world rate of 5% [4]. The enormity of the problem of ADR reporting and poor post marketing surveillance by the pharmaceutical companies in India has been well documented. Because of these factors, it is all the more important that a system of ADR reporting is established.
The reporting of ADRs has become an important component of the monitoring and the evaluation activities which are performed in hospitals. Since a major proportion of India’s population prefers government hospitals when they seek health care facilities, a good ADR database can be generated from these hospitals. A productive, hospital-based reporting program can also be instrumental in providing valuable information regarding the potential problems of drug usage in an institution. The most difficult task initially, is to foster a culture of reporting among the clinicians, especially among the junior doctors who have the most contact with the patients. The reasons for the low level of ADR reporting include lack of awareness, training, and most importantly, time. An additional factor is that the government has not made it mandatory for the health care providers to report ADRs, unlike some countries such as Spain and Sweden [5].
In November 2004, the Central Drugs Standard Control Organization, Ministry of Health and Family Welfare, Government of India, launched the National Pharmacovigilance Programme (NPP) which is expected to be successful, since this has been structured, taking into consideration the past deficiencies of similar efforts [6].
The present study was a humble attempt to set up a well organized ADR database generation reporting system in Goa Medical College and Hospital. All the reported ADRs were forwarded to the Hindu Pharmacy, Panjim, Goa, which is one among the 26 peripheral pharmacovigilance centres under NPP in India. As Goa Medical College is the only government tertiary care hospital in Goa, a productive hospital base reporting program can be instrumental in providing valuable information regarding the potential problems of drug usage in this institution. Through these efforts, the problems may be identified and resolved, which may result in continuous improvement in the patient care.
Materials and Methods
This was a prospective study which was conducted over a period of 15 months at Goa Medical College and Hospital (Goa, India), based on the ADRs which were reported from various departments of the hospital. It is a 2,200 bedded, only tertiary care hospital in the state which caters to all sections of the society. The approval of the institutional human ethics committee and permission from the head of the institution and the superintendent of the hospital were obtained before the commencement of the study.
We carried out an extensive education programme for all the clinicians and the interns on the importance of ADR reporting and provided them with a simple method for reporting the ADRs. Safely locked ‘ADR notification drop boxes’ [7], (with the key purpose printed on them) were installed systematically in all the clinical wards and selected Outpatient Departments (OPDs) in the hospital. Together with the boxes, ‘ADR notification forms’ which were designed on the basis of the WHO guidelines were kept [8]. The forms were also designed in such a way that the ‘notifier’ (doctor, intern, medical student or nurse) found it very easy to report an ADR.
The moment any health care provider suspected an ADR, a duly filled notification form was dropped in the ‘ADR notification drop box’. The suspected ADRs were carefully analyzed and documented at regular intervals. The drug manufacturer’s name was tracked down as per the brand details which were provided by the ‘notifier’. In the case of the hospital supply, the same was tracked down with assistance from the hospital pharmacy. On getting the required details, a red coloured “CDSCO Suspected ADR Reporting Form” which was provided by the pharmacovigilance centre, was completed.
All the relevant data which included the patients’ demographic details, diagnosis, suspected drugs which the patients received prior to the onset of the reaction, their respective dosage, their route of administration with frequency, the date of onset of the reaction, details of the reaction, any other concomitant drugs which were taken and finally, the name of the reporting doctor, the department to which he/she belonged to and the date of reporting were noted, selected and evaluated for the purpose of our study. Additional details on the ADRs were collected for evaluation purposes from the respective case records wherever they were required. The treating physician was approached wherever it was required, to get additional details and clarification. To ensure patient safety, each patient with an ADR was provided with an “ADR alert card” at the time of his/her discharge, who experienced such adverse reactions which by their nature, cautioned against the re-exposure of the suspected drug.
The monthly reports which were collected from the whole hospital were then forwarded to the Peripheral Pharmacovigilance Centre (Hindu Pharmacy, Panjim, Goa). Confidentiality was maintained at all the levels.
Evaluation of the data
The data on the reported ADRs were evaluated to understand their patterns, with respect to the notifier details, the patient demographics, the nature of the reactions and the characteristics of the drugs which were involved. The causality, severity and the outcome of the reactions were analyzed.
Notifier characteristics
The details of the clinical department to which the notifier belonged to and the time of reporting were also noted.
Patient characteristics
The patients’ age and sex were considered for evaluation. In agreement with the previous paper, [9], the patients were subdivided into six age groups; infants, children and adolescents (0–15 years), young adults (16–30 years), adults (31–45 years), older adults (46–60 years), elderly adults (61–75 years) and very elderly adults (over 75 years).
Reaction characteristics
The individual reactions were classified, depending on the type of reactions, as type A (Augmented) and type B (Bizarre) reactions, based on the classification which was done by Rawlins and Thompson [10]. The reactions were further classified, depending on the organ system which was affected.
Drug characteristics
The drugs which were responsible for the causation of ADRs were classified into drug classes. They were further classified, depending on their route of administration which was involved.
Analysis of the ADRs
Causality assessment
Each ADR was assessed for its causality by using the Naranjo Probability scale [11]. Dechallenge and rechallenge tests which were done to confirm the ADRs were done purely on the clinicians’ discretion.
Severity assessment
Depending upon the severity, the ADRs were classified into mild, moderate and severe reactions by using the criterion which was developed by Hartwig et al. for severity assessment [12].
Outcome assessment
The patient outcomes were reported as one of the following
Minor
Hospitalization (initial or prolonged)
Life threatening
Fatal
Results
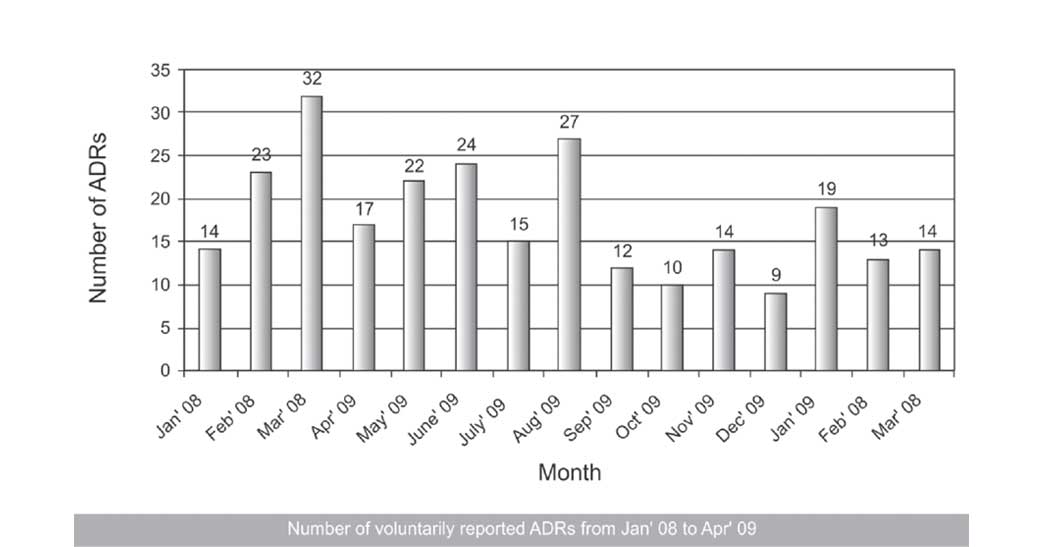
In the 15 month duration study that we conducted, a total of 265 ADRs were reported by the physicians from various clinical departments. The number of reported ADRs per month were variable, with an average of around 17 reports per month.
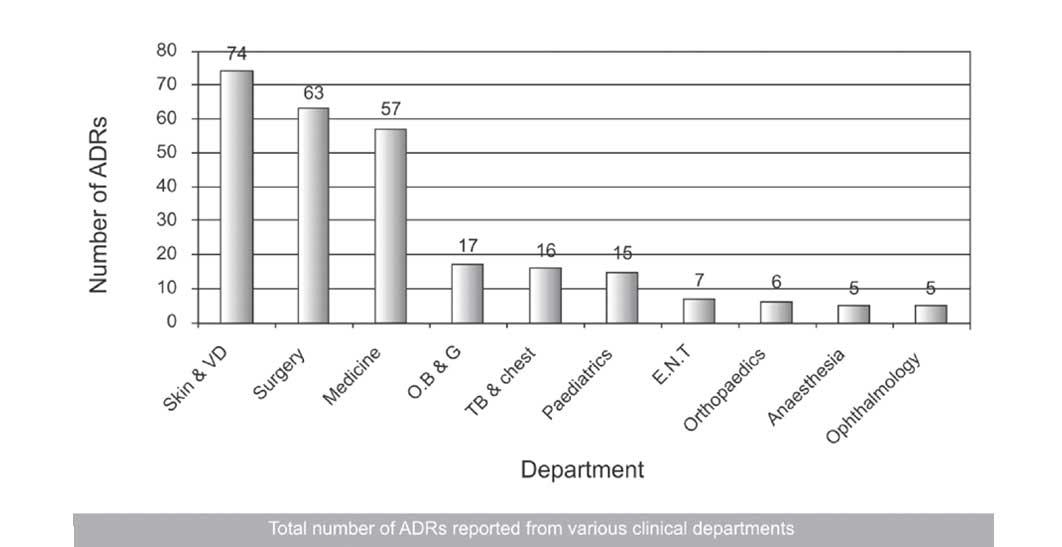
The Department of Dermatology reported the maximum number of ADRs (74, 27.92%), followed by the Departments of Surgery (63, 23.77%) and Medicine (57, 21.51%) [Table/Fig-1].
Total number of reported from various clinical departments
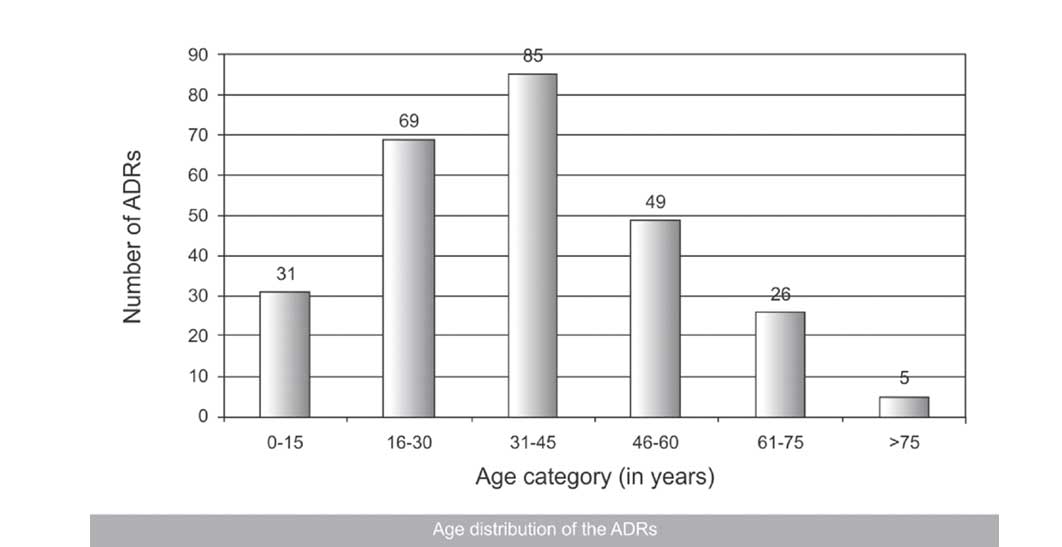
Females showed more ADRs (142, 54%) than males (123, 46%). The maximum number of reported ADRs were found in the adult group (85, 32.07%), followed by the young adult group (69, 26.03%) and the older adult group (49, 18.49%) [Table/Fig-2].
Age distribution of the ADRs
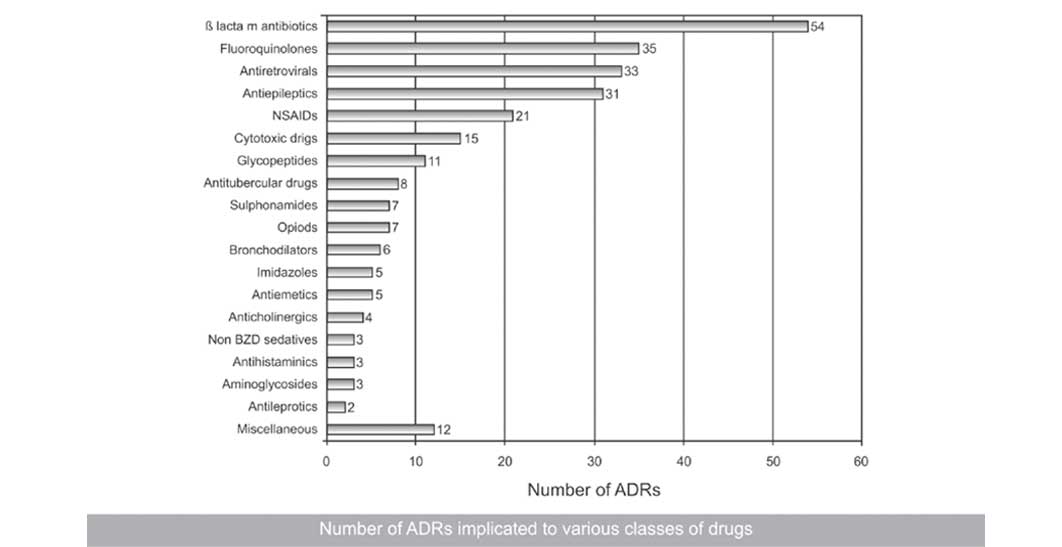
Among the drugs, the ß-lactam antibiotics were implicated the maximum number of times (54, 20.37%), followed by fluoroquinolones (35, 13.20%), antiretrovirals (33, 12.45%) and antiepileptics (31, 11.69%) [Table/Fig-3].
Number of ADRs implicated to various classes of drugs
Various organ system involved in reported ADRs
The comprehensive information which depicted the drugs and the nature of the adverse events, as was seen during the study is shown in [Table/Fig-4].
The skin was involved in about 57.73% (153) of the ADRs, while the CNS and the vascular system were involved in 8.67% (23) and 8.30% (22) of the ADRs [Table/Fig-5].
Depicting drugs and nature of adverse events seen during the
| Drug class | Number of ADRs | Individual drugs (number) | Adverse event |
|---|
| ß lactam antibiotics | 54 | Amoxicillin (10), ampicillin (1), aztreonam (4), benzathine penicillin (1), cefdinir (1), cefipime (1), cefotaxime (1), cefpodoxime proxetil (2), ceftazidime (8), ceftrioxone (2), cefuroxime (5), cefuroxime axetil (5), piperacillin tazobactum (13) | Hypersensitivity skin reactions, Fixed Dose Eruptions (FDEs), giddiness, blurring of vision, vomiting, nicalau syndrome, lid edema, bradycardia, chills, rigors, tinnitus, Steven Johnson Syndrome (SJS), anaphylactic shock, haemetemesis, tachycrdia, diarrhoea, bitter taste. |
| Fluoroquinolones | 35 | Indinavir (2), lamivudine (2), nevirapine (19), zidovudine (10) | Hypersensitivity skin reactions, Erythema Multiforme (EM), lymphadenopathy, target lesions, oral erosions, acneiform eruptions, jaundice, SJS, anemia, hepatitis. |
| Antiepileptics | 31 | Carbamazepine (12), phenobrbitone (1), phenytoin (18) | Hypersensitivity skin reactions, exfoliative dermatitis, target lesions, conjuctival congestion, fever, SJS, gum hypertrophy, lip bleeding, jaundice, EM, Toxic Epidermal Necrolysis (TEN) |
| NSAIDs | 21 | Diclofenac sodium (4), etoricoxib (1), ibuprofen (6), mafenemic acid (1), nimesulide (5), paracetamol (4) | Hypersensitivity skin reactions, acute renal failure, malena, insomnia, gastrointestinal tract perforation, upper gastric bleed, EM |
| Cytotoxic drugs | 15 | 5 fluorouracil (6), azathioprine (2), capacitabine (1), cisplatin (1), cyclophosphamide (1), doclitaxel (2), methotrexate (1), paclitaxel (1) | Neutropenia, agranulocytosis, thrombophlebitis, pancytopenia, hand and foot syndrome, loss of hair, cystitis, generalized pigmentation, oral erotions, diarrhoea, vomitting, hypersensitivity kin reactions |
| Glycopeptides | 11 | Vancomycin (11) | Hypersensitivity skin reactions, red man syndrome, chills, rigors. |
| Antitubercular drugs | 8 | Ethambutol (1), isoniazid (5), rifampicin(2) | Peripheral neuropathy, hypersensitivity skin reactions, drug fever, hepatitis, anaphylactic shock |
| Sulphonamides | 7 | Cotrimoxazole (7) | Hypersensitivity skin reactions, SJS, TEN, FDEs |
| Opiods | 7 | Morphine (1), tramadol (6) | Constipation, nausea, vomiting, hypersensitivity skin reactions |
| Bronchodilators | 6 | Aminophylline (3), deriphylline (1), salbutamol (2) | Seizure, epigastric pain, irregular pulse, palpitations, coarse tremors. |
| Imidazoles | 5 | Metronidazole (4), tinidazole (1) | Nausea, vomiting, hypersensitivity skin reactions, hypotension |
| Antiemetics | 5 | Domeperidone (2), ondensetron (3) | Sedation, bradycardia, tachycardia |
| Anticholinergics | 4 | Atropine (4) | Urinary retention, Dribbling of urine |
| Non BZD hypnotics | 3 | Zolpidem (3) | Psychosis, Tremors, Ataxia, Forgetfulness, Nocturnal Sleep Related Eating Disorder (NSRED) |
| Antihistaminics | 3 | Cetirizine (1), loratidine (1), promethazine (1) | Sedation, Hypersensitivity skin reactions. |
| Aminoglycosides | 3 | Amikacin (3) | Nephrotoxicity, Hypersensitivity skin reactions |
| Antileprotics | 2 | Clofazimine (1), dapsone (1) | Hypersensitivity skin reactions, oral erotions |
| Miscellaneous | 12 | Azithromycin (1), carbimazole (1), clindamycin (2), fluconazole (2), hydrocortosone (1), linezolid (1), olanzapine (1), pentoxiphylline (1), propofol (1), pseudoephedrine (1) | Giddiness, slurred speech, aplastic anemia, hypersensitivity skin reactions, hypotension, lip erosions, anaphylactic shock, nausea, vomiting, EM, lid edema, rigors, insomnia |
The oral route was responsible for the ADR causation in 63.39% (168) cases as compared to the intravenous/intramuscular route (90, 33.96%). Most of the ADRs were categorized as “Type II” (203, 77%) against “Type I” (62, 23%) by the Rawlins and Thompson’s classification. The causality assessment was done by the Naranjo Algorithm and 62.26% (165) ADRs were found to fall in the “probable category” as compared to 29.05% (77) in the “highly probable” one.
Among the all ADRs which were reported, 34.71% (148) were “severe”, in accordance with the Modified Hartwig and Siegel’s scale. Among all the ADRs which were reported, 7.92% (21) were life threatening. Altogether, 10 (3.77 %) fatalities were observed.
Discussion
In our study, the total numbers of spontaneously reported suspected ADRs in our government hospital setting were 265 in a span of 15 months, with an average of around 17 ADRs per month. This finding was similar to that of one of the studies which was done in south India by Arulmani et al., where the authors found the average number of spontaneously reported suspected ADRs in their hospital to be 20/month [13]. But our finding was not in line with those of the studies which were done by Diskhit et al.,[14] and Jose et al.,[15] who found the average to be 28 and 34 per month respectively. This finding can be attributed to the fact that neither had we had a streamlined ADR reporting system in our hospital in the past, nor was our hospital a part of the pharmacovigilance set up under NPP, whereas both the above mentioned studies were done in hospitals which were actually among the 26 peripheral pharmacovigilance centres which were under NPP. They benefited by organizing timely Continuous Medical Education (CME) programs for their doctors and inter departmental meetings to increase the awareness of the physicians about the importance of ADR monitoring. This point was also favoured by the fact that the average number of spontaneously reported ADRs was only 9/month in the study which was done by Baniasadi et al., in Iran, where the authors introduced the concept of pharmacovigilance for the first time in their teaching hospital [16]. As far as the various clinical departments were concerned, the Department of Dermatology reported the maximum number of ADRs (74, 27.92%), followed by the Departments of Surgery (63, 23.77%) and Medicine (57, 21.51%). The least were reported from the Departments of Anesthesia and Ophthalmology (5, 1.88% each).
The demographic details of our study showed a female gender predominance over males for ADRs, (F-54%, M-46%) which was similar to that which was found in other studies which have been reported in the literature [17,18], though on the other hand, some studies have been published, where males were implicated more as compared to females [19,20].
Previous studies have shown that a larger percentage of ADRs were reported from the geriatric and the paediatric populations, which had no similarity with our results [21]. Age-related changes in the pharmacokinetics and the pharmacodynamics of the drugs seem to be the possible explanation of the higher incidence of ADRs in these two groups [22]. On the contrary, our study revealed that the maximum number of reported ADRs was found in the adult group (31-45 years, 32.07%), followed by the young adult group (16-30 years, 26.03%) and the older adult group (46-60 years, 18.49%). These results were congruent with only a few published data [16]. Possibly, a higher percentage of patients from the young age group who reported to the hospital and the under-reporting of ADRs could be few of the reasons for these kind of results in our scenario.
Among the drugs, antimicrobials, mainly the ß-lactam antibiotics, fluroquinolones, glycopeptides, antitubercular, imidazoles and others accounted altogether for 48.67% (129) of all the reported ADRs. The other important ones included antiretrovirals (33, 12.45%), antiepileptics (31, 11.69%), Nonsteroidal Anti-inflammatory Drugs (NSAIDS) (21, 7.92%) and cytotoxic drugs (15, 5.66%). These findings were very much consistent with those of other studies where antimicrobials were responsible for the maximum causation of ADRs [23,24]. There are only few studies that have shown cytotoxic agents [25] or NSAIDS [26] to be maximally involved in causing ADRs.
The dermatological system (153, 57.72%) was the organ system which was most commonly affected by the ADRs in our study, followed by the neurological (23, 8.67%) and the cardiovascular systems (22, 8.30%). These findings went hand in hand with those of other studies where skin involvement was seen in a maximum number of cases among the reported ADRs [27]. Neurological ADRs too had been on the top of the list of ADRs in previous studies [28]. The gastro-intestinal system was reported to be most commonly involved in few of the published data [29]. In our study, this formed the fourth largest report on ADRs.
The oral route was responsible for the ADR causation in 63.39% (168) cases as compared to the intravenous/intramuscular route (90, 33.96%). This was at par with the findings of a study which was done by Sharma et al., [20] Not many studies took this point into consideration as one of the drug characteristics in ADR monitoring. The more common intake of oral drugs as compared to parenteral ones could be one of the reasons for this particular finding.
Most of the ADRs were categorized as “Type B” (203, 77%) against the “Type A” (62, 23%) reactions, the results being not consistent as was proposed by Rawlins and Thompson’s classification. Very few studies supported our finding in this context.19 Most of the published data showed that the “Type A” reactions were commonly reported than the “Type B” reactions [30]. However, the ease in diagnosing hypersensitivity and anaphylactic reactions and the high severity may be able to inspire the physicians in reporting such reactions more commonly. Also, the large number of reactions which were reported for antibiotics, which usually are “Type B” in nature, must have contributed to this higher share of “Type B” reactions in our study.
Most of the reactions belonged to the category ‘probable’ (165, 62.26%), based on the causality assessment, which was similar to the results of another study [13,15] but it was different from the results which were observed by Murphy and Frigo [31] in which more possible reactions were noticed. Considering the severity of the reactions, a majority of the reactions were found to be mild in severity (148, 55.84), which was similar to the results which were published in other studies [13], but it was different from the results of certain other studies [32] wherein more moderate reactions were observed. Quite a major percentage of the reactions (92, 34.71%) were severe in nature and mostly skin reactions accounted for the same. Patients getting admitted in the hospital or their stay getting prolonged were the main reasons for so many ADRs falling into this “severe” category as per the severity assessment scale.
As far as the outcome of the reported ADRs was concerned, a chunk of them was found to lead to an initial or prolonged hospitalization of the patients (87, 32.83%), as compared to other studies which did not show such a higher percentage [13]. Twenty one (7.83%) of them were life threatening and unfortunately, 10 fatalities were seen during the study time period. The main causes of these deaths were anaphylactic shock which was caused by the β-lactam antibiotics, fatal skin reactions (Steven Johnson Syndrome and Toxic Epidermal Necrolysis) which were caused by antiepileptics, cytotoxic drugs induced pancytopaenia and NSAIDs induced gastrointestinal perforation. These findings were somewhat similar to those of a large drug related death analysis of an Italian spontaneous reporting database, where they found that ‘systemic anti-infective drugs’ was the drug category which was associated with the highest percentage of fatal ADRs, followed by antineoplastic agents. Serious skin or systemic allergic reactions were the main causes of these deaths, which was similar to that which we found in our study [33].
Conclusion
The present work was a humble attempt in setting up a well organized ADR database generation reporting system at our government hospital. The systematic tracking and monitoring of ADRs can shed light on their extensiveness and their patterns of occurrence. A similar data evaluation needs to be followed by the dissemination of the information to the healthcare professionals, which can help in improving the quality of the patient care by ensuring the safer use of drugs. The data which was obtained will be useful for the future, long term and for a more extensive ADR monitoring in our hospital and for the promotion of rational prescribing and drug use in our hospital.
To get sustained results from this strategy for reporting ADRs, there has to be a strong collaboration between the Department of Pharmacology and other clinical departments. ADR monitoring could be made a compulsory part of the training for postgraduate students and as a part of their M.D. curriculum. The interns can be taught about ADR reporting during their Internship Orientation Programme, so that they too can assist the resident doctors who work in various clinical departments. Lectures can be taken for the undergraduates on the importance of pharmacovigilance and ADR reporting. The students could be given an exercise, such as to report three ADRs in their term, which they can do during their ward postings. Pharmacovigilance awareness programmes can be imparted to all the nurses and other allied health staff working in the hospital. This strategy, if adopted by all the government hospitals and medical colleges, could be a useful stepping stone for generating a genuine ADR database for our Indian population.
[1]. Esch AF, The planning of a national drug monitoring system. A WHO.Technical Report Series 1972 498:44-7. [Google Scholar]
[2]. Pourpak Z, Fazlollahi MR, Fattahi F, Understanding adverse drug reactions and drug allergies: principles, diagnosis and treatment aspects.Recent Pat Inflamm Allergy Drug Discov 2008 2(1):24-46. [Google Scholar]
[3]. Einarson TR, Drug-related hospital admissions.Ann Pharmacother 1993 27:832-40. [Google Scholar]
[4]. Prakash S, Pharmacovigilance in India.Indian J Pharmacol 2007 39:123-3. [Google Scholar]
[5]. Brewer T, Colditz GA, The postmarketing surveillance and adverse drug reactions: the current perspectives and future needs.JAMA 1999 281(9):824-9. [Google Scholar]
[6]. Adithan C, The National Pharmacovigilance Program.Indian J Pharmacol 2005 37:347 [Google Scholar]
[7]. Amit D, Rataboli PV, The adverse drug reaction (ADR) notification drop box: an easy way to report ADRs.Br J Clin Pharmacol 2008 66(5):723-4. [Google Scholar]
[8]. Kurokawa T, Correa-Nunes AM, Czarnecki A, Guidelines for setting up and running a Pharmacovigilance Centre. 2000 SwedenUppsala Monitoring Centre, WHO Collaborating Centre for International Drug Monitoring:4-10. [Google Scholar]
[9]. Gallelli L, Ferreri G, Colosimo M, Pirritano D, Guadagnino L, Pelaia G, Maselli R, De Sarro GB, Adverse drug reactions to antibiotics which were observed in two pulmonology divisions of Catanzaro, Italy: a six year retrospective study.Pharmacol Res 2002 46(5):395-400. [Google Scholar]
[10]. Rawlins M, Thompson W, Davies D, Mechanisms of adverse drug reactionsTextbook of adverse drug reactions 1991 New YorkOxford University Press:18-45. [Google Scholar]
[11]. Naranjo CA, Busto U, Sellers EM, Sandor P, Ruiz I, Roberts EA, Janecek E, Domecq C, Greenblatt DJ, A method for estimating the probability of adverse drug reactions.Clin Pharmacol Ther 1981 30:239-45. [Google Scholar]
[12]. Hartwig SC, Siegel J, Schneider PJ, Preventability and severity assessment in reporting adverse drug reactions.Am J Hosp Pharm 1992 49:2229-2232. [Google Scholar]
[13]. Arulmani R, Rajendran SD, Suresh B, Adverse drug reaction monitoring in a secondary care hospital in south India.Br J Clin Pharmacol 2007 65(2):210-216. [Google Scholar]
[14]. Dikshit RK, Desai C, Desai MK, The pleasures and the pains of running a pharmacovigilance center.Indian Pharmacol 2008 40:31-S34. [Google Scholar]
[15]. Jose J, Rao PG, The pattern of adverse drug reactions which was notified by spontaneous reporting in an Indian tertiary care teaching hospital.Pharmacol Res 2006 54:226-33. [Google Scholar]
[16]. Baniasadi S, Fahimi F, Shalviri G, Developing an adverse drug reaction reporting system at a teaching hospital.Basic Clin Pharmacol Toxicol 2008 102(4):408-411. [Google Scholar]
[17]. Ramesh M, Pandit J, Parthasarathi G, Adverse drug reactions in a south Indian hospital – their severity and the costs which were involved.Pharmacoepidemiol. Drug Saf 2003 12:687-92. [Google Scholar]
[18]. Hoddinott BC, Drug reactions and errors in the admissions to a medical ward.Can Med Assoc J 1967 97:1001-6. [Google Scholar]
[19]. Subbanna PKT, Chandy SJ, The role of active surveillance in improving the hospital adverse drug event reporting.Indian J Pharmacol 2006 38(5):363-4. [Google Scholar]
[20]. Sharma H, Aqil M, Imam F, Alam MS, Kapur P, Pillai KK, A pharmacovigilance study in the department of medicine of a university teaching hospital.Pharmacy Practice 2007 5(1):46-9. [Google Scholar]
[21]. Somers A, Petrovic M, Robays H, Bogaert M, Reporting the adverse drug reactions in a geriatric ward: a pilot project.Eur J Clin Pharmacol 2003 58:707-14. [Google Scholar]
[22]. Ciorciaro C, Hartmann K, Kuhn M, Differences in the relative incidences of adverse drug reactions in relation to age. An evaluation of the spontaneous reporting system of SANZ:Journal Suisse de medicine 1998 128:254-8. [Google Scholar]
[23]. Prosser TR, Kamysz PL, A multidisciplinary adverse drug reaction surveillance program.Am J Hosp Pharm 1990 47(6):1334-39. [Google Scholar]
[24]. Uppal R, Jhaj R, Malhotra S, Adverse drug reactions among the inpatients in a north Indian referral hospital.Natl Med J India 2000 13(1):16-8. [Google Scholar]
[25]. Olivier P, Boulbes O, Tubery M, Carles P, Montastruc JL, Lapeyre-Mestre M, Preventability of the adverse effects in a medical emergency service.N Engl J Med 2001 56(3):275-8. [Google Scholar]
[26]. Polimeni G, Salvo F, Cutroneo P, Morreale I, Patrizio Caputi A, Adverse reactions which were induced by NSAIDs and antibacterials: an analysis of spontaneous reports from the Sicilian regional database.Drug Saf 2006 29(5):449-459. [Google Scholar]
[27]. Classen DC, Pestotnik SL, Evans RS, Burke JP, A computerised surveillance of the adverse drug events in hospitalized patients.JAMA 1991 266:2847-51. [Google Scholar]
[28]. Gurwitz JH, Field TS, Avorn J, McCormick D, Jain S, Eckler M, Benser M, Edmondson AC, Bates DW, The incidence and the preventability of adverse drug events in nursing homes.Am J Med 2000 109:87-94. [Google Scholar]
[29]. Suh DC, Woodall BS, Shin SK, Hermes-de-Santis ER, The clinical and the economic impact of the adverse drug reactions in hospitalised patients.Ann Pharmacother 2000 34:1373-79. [Google Scholar]
[30]. Laurence DR, Bennett PN, Brown MJ, Unwanted effects and adverse drug reactionsClinical Pharmacology9th ed.:135-50. [Google Scholar]
[31]. Murphy BM, Frigo LC, The development, implementation and the results of a successful multidisciplinary adverse drug reaction reporting program in a university teaching hospital.Hosp Pharm 1993 28:1199-204. [Google Scholar]
[32]. Gholami K, Shalviri G, The factors which are associated with the preventability, predictability, and the severity of adverse drug reactions.Ann Pharmacother 1999 33(2):236-40. [Google Scholar]
[33]. Leone R, Sottosanti L, Luisa Iorio M, Santuccio C, Conforti A, Sabatini V, Moretti U, Venegoni M, Drug-related deaths: an analysis of the Italian spontaneous reporting database.Drug Saf 2008 31(8):703-713. [Google Scholar]