Introduction
An ocular inflammation results from various causes; traumatic and nontraumatic. The traumatic causes may be surgical or non-surgical. The nontraumatic causes include infective, immunological, chemical and radiological causes, etc [1].
A non-infectious postoperative ocular inflammation may occur, following various ocular surgical procedures like cataract surgery, strabismus surgery, trabeculoplasty, refractive surgery, penetrating keratoplasty, etc [2–4]. The physical trauma which is associated with an ocular surgery may induce an inflammatory response due to the release of various inflammatory mediators and the recruitment of neutrophils and macrophages. The prostaglandins (PGs) which are mainly involved as the inflammatory mediators also disrupt the Blood Aqueous Barrier (BAB), they produce changes in the intraocular pressure and they cause intraoperative miosis [5]. Cataract surgeries (extra capsular cataract extraction) are frequently associated with postoperative ocular inflammation because of the tissue injury which releases PGs from the uveal tissues and pigment material from the traumatized iris [6]. Although the recent technical advances and the refinements in cataract surgeries such as phacoemulsification techniques, small incision surgeries, and foldable Intraocular Lenses (IOLs), have significantly decreased the extent of the ocular injury, they have not totally eliminated the trauma-induced synthesis and the release of inflammatory mediators. Therefore, most of the patients still experience some degree of postoperative inflammation, pain, or both after the surgery [7].
Commonly, corticosteroids are used for the control of the inflam-mation but these steroids are associated with adverse reactions, both acute and chronic, like an increased Intraocular Pressure (IOP)-glaucoma, a posterior subcapsular cataract, a delay in/re-tardation of the wound healing, a decreased wound strength, an increased susceptibility for infections (bacterial ,viral, fungal and parasitic), ptosis, exophtholmus, pseudotumour cerebri, keratocyte apoptosis, corneal melting syndrome, scleromalacia perforanse, scleral staphyloma, crystalline keratopathy, extrocular muscle imbalance and a retinal or a choroidal embolic phenomenon [8,9].
Hence, this prospective, open label, interventional study was done to compare the efficacy and the tolerability of the topical NSAID, Flurbiprofen with those of the topical steroid -Loteprednol in the post-operative inflammation.
Methodology
After obtaining the approval of and clearance from the institutional ethical committee, 40 patients (20 in the flurbiprofen group and 20 in the Loteprednol group) with senile cataracts, who were undergoing small incision suture less cataract surgeries with posterior chamber intra ocular lens implantation (SISCS-PCIOL) were recruited for the study. All the study medications were purchased from the local hospital pharmacy and the prednisolone eye drop was kept as the rescue medication for any untoward effect. No study related procedures were started before taking a written informed consent from the participants. The procedures which were followed during the study were in accordance with the ethical standards which were laid down by Indian Council of Medical Research (ICMR)'s guidelines for biomedical research on human subjects (2006) and with the Declaration of Helsinki 1975 –which was revised in 2000.
The inclusion and the exclusion criterion were as follows;
Inclusion Criteria
The patients of either gender who had undergone SISCS-PCIOL- uncomplicated
Exclusion Criteria
The patients with known / suspected allergy to the NSAIDs
The patients who developed intra-operative complications
A pre-existing ocular inflammation
A previous intraocular surgery
The patients who received other topical medications
One-eyed individuals
The patients with IDDM
The patients who were aged < 25 years
The patients who had used NSAIDs in the past 2 weeks
The Study Procedure
A written informed consent was obtained from the participants after fully explaining to them, the procedure and its consequences and the right to withdraw at any point of period, in their own languages.
The study medications were administered by the instillation of 1 drop into the operated eye, 4 times daily for 28 days, starting from the day of the surgery. Tropicamide eye drops (1%) was used for all the patients preoperatively, 1-2 drops every 30 minutes for 2 hours. The patients also received gatifloxacin eye drops (0.3%) 4 times daily, starting a day before the surgery and it was continued for 4 weeks. The patients were examined on the days - 1, 7, 14, 21 and 28 after the surgery, and grading for the inflammation was done by using a 4-point scale which ranged from 0-3. The analgesia was graded by the patient's subjective assessment of the post-operative pain/discomfort by using a visual analogue scale (which was graded as 0-10).
Grading: Aqueous flare (slit lamp examination) [4]
Grade 0 – Absent, Grade 1– Mild (barely detected), Grade 2– Moderate (iris and lens details seen) and Grade 3– Severe (iris and lens not visible).
Cells in the anterior chamber (slit lamp examination) [10]
Grade 0– None
Grade 1– 1-15 cells
Grade 2–15-25 cells
Grade 3– >25cells
Corneal odema (slit lamp examination) [4]
Grade 0– absent
Grade 1– Mild
Grade 2–Moderate
Grade 3–severe
Conjunctival congestion [4]
Grade 0– None
Grade 1– Mild (some vessels injected)
Grade 2–Moderate (diffusely injected)
Grade 3– Severe (intense injection)
Ciliary congestion
Grade 0– Nil
Grade 1– Mild (the presence of a ciliary flush which is visible on the slit lamp examination)
Grade 2–Moderate (ciliary congestion which is visible with the naked eye)
Grade 3–Severe (intense congestion)
Analgesia [11]
The analgesia was graded by the patient's subjective assessment of the post-operative pain/discomfort by using a visual analogue scale (VAS) which was graded as 0-10, where 0 = no pain and 10 = worse imaginable pain.
Dosage: The study drugs were instilled into the affected eye, one drop four times a day – for the respective patient groups for 4 weeks. The patient compliance was monitored by using the “daily drug reminder chart” during the follow-up visits. Follow up examinations were done on the 7th, 14th, 21st and the 28th days to assess/grade the study parameters.
Results
At the end, all the patients were included in the analysis, as there were no dropouts from the study. All the patients’ inflammatory scores were found to be comparable at the baseline on the use of the single unpaired t test.
The demographic characteristics: the mean age of the pooled patient population was 64.55+ 6.33, the groups were age matched (with P=0.311) and the gender distribution was statistically similar between the groups (with P=0.109).
The study parameters like the aqueous-flare, cells in the anterior chamber, ciliary congestion and corneal oedema were of mild grade as has been depicted in the [Table/Fig-1], which subsided by visit-3 and were statistically comparable in both the groups. Whereas, the conjunctival hyperaemia persisted up to visit-4 in a mild form in both the groups and it was statistically comparable, as has been depicted in [Table/Fig-2].
The subjective parameter, ocular pain, as was assessed by using VAS, was present till visit 4 and it was comparable in both the groups.
Mean Scores – Subjective & Objective Parameters
| Group I Flurbiprofen | Group II Loteprednol | P value | T test |
| Aqueous flare |
| Visit 1 | 0.95±0.51 | 1±0.56 | 0.770 | 0.295 |
| Visit 2 | 0.35±0.49 | 0.3±0.47 | 0.714 | 0.370 |
| Visit 3 | 0 | 0 | - | - |
| Visit 4 | 0 | 0 | - | - |
| Visit 5 | 0 | 0 | - | - |
| Cells in anterior chamber |
| Visit 1 | 1.15±0.58 | 1.05±0.6 | 0.599 | 0.531 |
| Visit 2 | 0.2±0.41 | 0.3±0.47 | 0.744 | 0.330 |
| Visit 3 | 0 | 0 | - | - |
| Visit 4 | 0 | 0 | - | - |
| Visit 5 | 0 | 0 | - | - |
| Corneal edema |
| Visit 1 | 0.3±0.47 | 0.45±0.51 | 0.520 | 0.650 |
| Visit 2 | 0.05±0.22 | 0 | 0.324 | 1.000 |
| Visit 3 | 0 | 0 | - | - |
| Visit 4 | 0 | 0 | - | - |
| Visit 5 | 0 | 0 | - | - |
| Conjunctival hyperaemia |
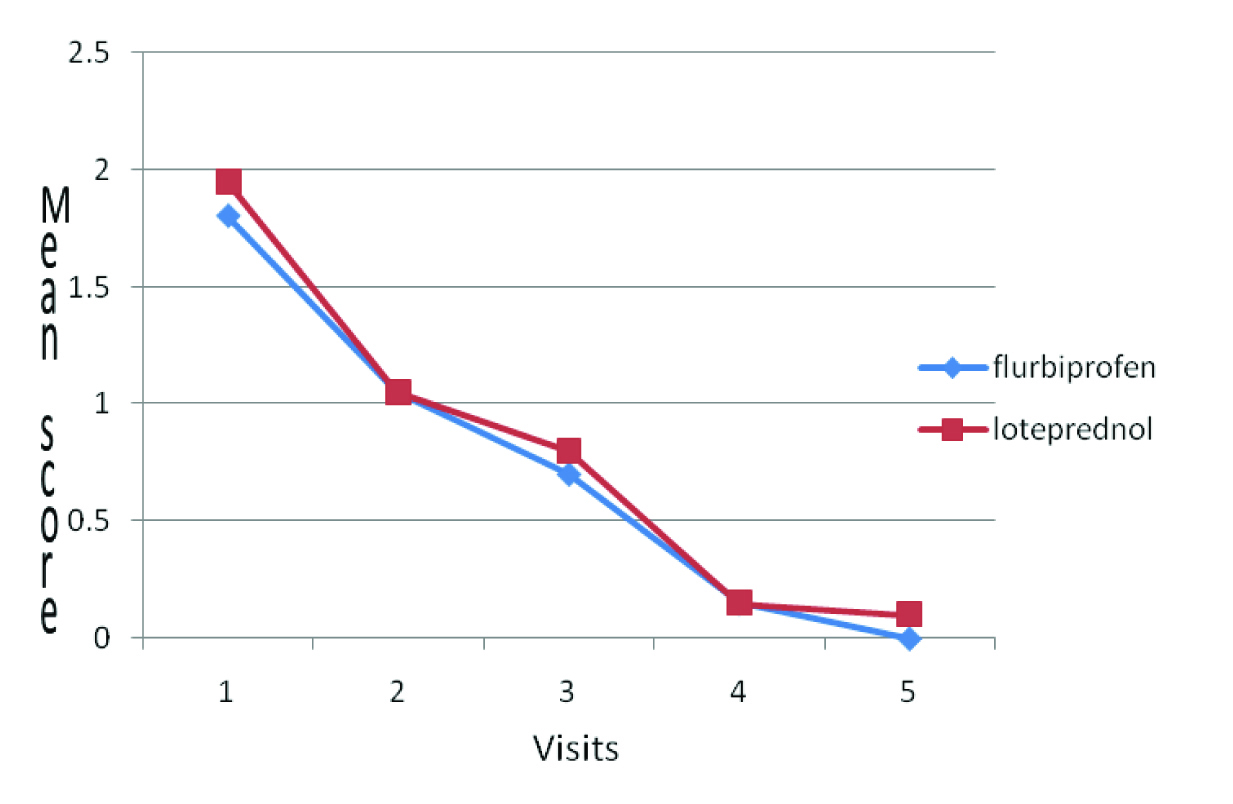
| Visit 1 | 1.95±0.51 | 1.8±0.41 | 0.312 | 1.024 |
| Visit 2 | 1.05±0.22 | 1.05±0.22 | 1.000 | 0.00 |
| Visit 3 | 0.8±0.41 | 0.7±0.47 | 0.478 | 0.717 |
| Visit 4 | 0.15±0.37 | 0.15±0.37 | 1.000 | 0.000 |
| Visit 5 | 0.1±0.31 | 0±0 | 0.154 | 0.1453 |
| Ciliary congestion |
| Visit 1 | 1±0.32 | 1.25±0.44 | 0.324 | 1.024 |
| Visit 2 | 0.3±0.47 | 0.5±0.51 | 0.520 | 0.650 |
| Visit 3 | 0 | 0 | - | - |
| Visit 4 | 0 | 0 | - | - |
| Visit 5 | 0 | 0 | - | - |
| Pain (VAS) |
| Visit 1 | 4.45±0.89 | 4.55±0.76 | 0.704 | 0.383 |
| Visit 2 | 2.1±0.97 | 1.35±0.81 | 0.053 | 2.000 |
| Visit 3 | 0.9±0.55 | 0.65±0.59 | 0.389 | 0.872 |
| Visit 4 | 0.25±0.44 | 0 | - | - |
| Visit5 | 0 | 0 | - | - |
Conjunctival Hyperaemia mean-score comparison
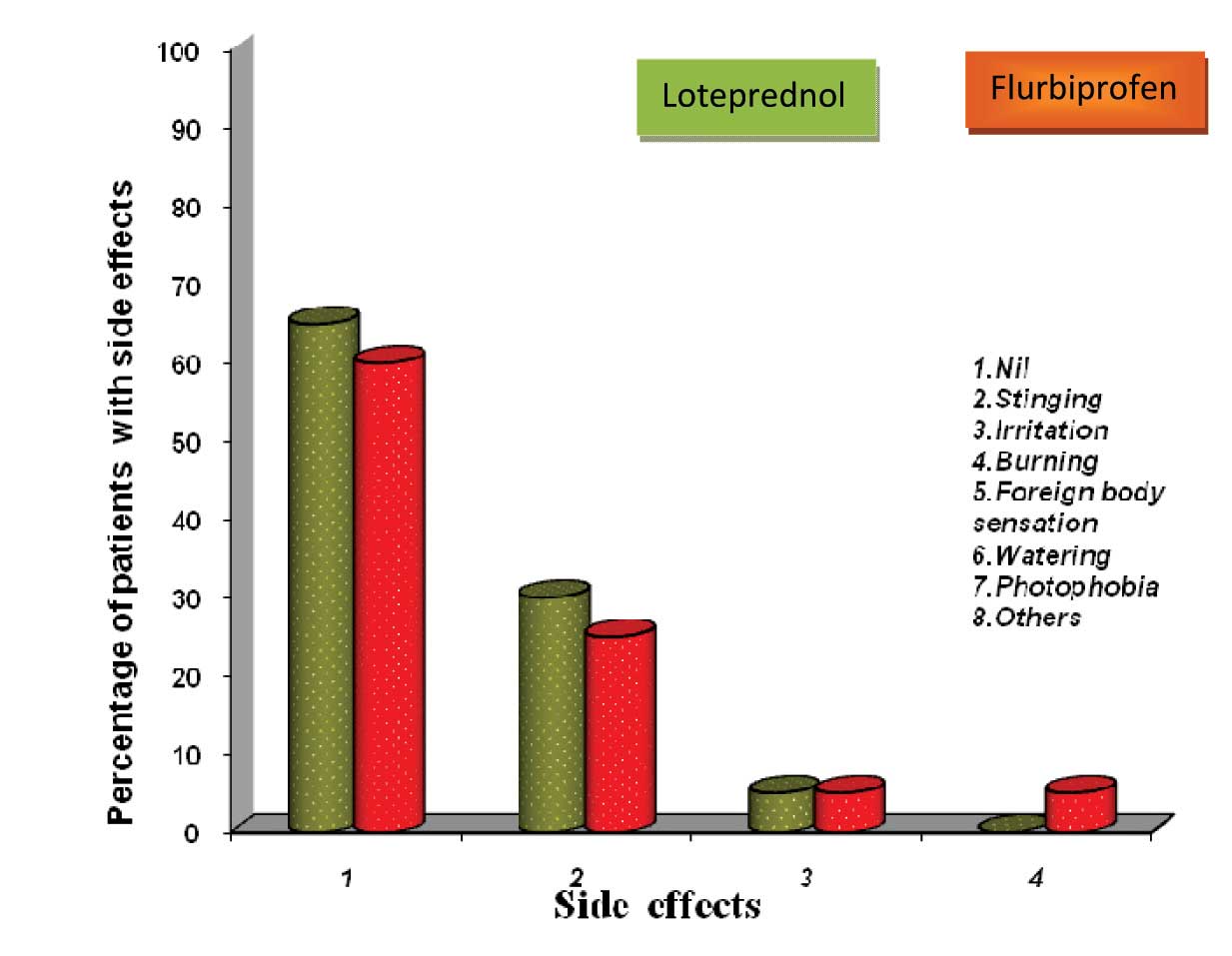
All the patients showed good compliance and they tolerated the medications. No serious adverse reactions were reported, as has been depicted in [Table/Fig-3]. The mild stinging and the irritation which were experienced by the patients after the instillation of the medications, were of a transient nature and they were self-limited.
Side effect profile-comparison
Discussion
A wide range of anti-inflammatory drugs are available to suppress ocular inflammations, usually with the use of a topical application and occasionally with the use of a systemic administration. These include steroids, NSAIDs, antihistaminics, mast cell stabilizers and immunomodulators.
The steroidal anti-inflammatory agents include natural, semisynthetic and synthetic glucocorticoids with powerful and nonspecific anti-inflammatory, antiallergic and immunosuppressant actions which result as the overall consequence of multiple mechanisms of action, which are mediated through specific intracellular receptors, which modulate the gene expression and affect all the components of the inflammatory and the immune responses [12]. These are seen as, a reduction in the recruitment and the activation of the inflammatory cells, a decrease in the release of various cytokines (ILs, IFNs, TNF α, PAF, etc.), inhibition of phospholipase A2 by inducing lipocortin synthesis, a decrease in the synthesis of arachidonic acid and its derivative mediators (PGs and LTs), a decrease in the expression of COX-2 and NOS-2, a decrease in the synthesis of the adhesion molecules (ElLAM-1, ICAM-1) and a decrease in the T-lymphocyte activation and proliferation. However, the actions of steroids are nonspecific and palliative but not curative, thus suppressing the tissue component of the inflammatory and the autoimmune mechanisms without eradicating the cause.
Steroids were first introduced into the ocular therapy in 1950: the topical formulations as eye drops and the injectable formulations of cortisone acetate in 1951 [8].
In the ophthalmic practice, glucocorticoids are used through various routes, depending upon the site of involvement and the desired steroid concentration at the target tissue or site. Glucocorticoids are readily absorbed from the cornea, conjunctiva and the sclera. However, the ocular penetration of the steroids occurs mainly through the cornea and it depends upon the relative water and the lipid solubility and the particle size, their concentrations, viscosity, pH and tonicity, the presence of other additives (adjuvants, preser-vatives-methylcellulose, etc), and the condition of the corneal epithelium [8].
The ocular adverse effects of the steroids depend upon the dose and the duration of the steroid therapy, the potency of the steroid, the underlying disease states and the patient susceptibility. In addition, the systemic absorption of the topically applied steroids may also produce systemic adverse effects.
The steroid induced/related ocular adverse effects and the complications include; increased Intraocular Pressure (IOP)-glaucoma, a posterior subcapsular cataract, a delay in/retardation of the wound healing, a decreased wound strength, an increased sus-cep-tibility for infections, mydriasis, ptosis, exophtholmus, pseudotumor cerebri, keratocyte apoptosis, corneal melting syndrome, scler-omalacia perforanse, scleral staphyloma, crystalline keratopathy, extrocular muscle imbalance and a retinal or a choroidal embolic phenomenon [8,9].
Hence, in this study, the steroid, Loteprednol was compared with the topical NSAID, Flurbiprofen.
Loteprednol, a ‘soft’ steroid, is less likely to cause steroid related ocular complications, while preserving the useful anti-inflammatory action. Loteprednol is an ester glucocorticoid with a 17-β chloro-methyl ester group and it is rapidly de-esterified to inactive the metabolites in the corneal tissue [2,13]. Hence, it produces lesser systemic adverse effects.
The currently available NSAIDs for topical application on the eye include; flurbiprofen diclofenac, indomethacin, suprofen, ketorolac, nepafenac, bromofenac and amfenac [5].
The anti-inflammatory action of the NSAIDs is primarily due to the inhibition of the cyclooxygenase enzyme (COX-1 and COX-2) and due to a decrease in the biosynthesis and the release of the proinflammatory PGs- PGE2, PGF2α PGD2 and PGI2 [14]. Additional mechanisms like suppressing the leukocyte motility and chemotaxis, inhibiting the inflammatory cytokines and the free radical scavenging activity, may also contribute to their anti-inflammatory action [5].
The topical NSAIDs provide certain distinct advantages over the topical steroids in ocular inflammation. Apart from being virtually free from the steroid related complications like cataract, glaucoma, an increased susceptibility for infections and a delayed wound healing, they also effectively prevent intraoperative miosis and protect against cystoid macular oedema. In addition, they are more effective than the topical steroids in reestablishing BAB [5].
In the earlier studies which were done, the topical NSAIDs like flurbiprofen ketorolac, bromofenac and diclofenac had been evalu-ated for their anti-inflammatory actions and the prevention of intraoperative miosis and cystoids macular oedema [15–18].
Most of the published studies had been done in Caucasians and our study was done in the Indian population. We compared the soft steroid Loteprednol with an NSAID. Our results were comparable with those of the western studies, but our study population included only the patients with an uncomplicated form of the post-operative inflammation and the sample size was 40. Hence, there is a need to study a large number of patients in order to have more data and to validate these conclusions. However, steroids being powerful anti-inflammatory agents , they can be preferred in the patients with a severe form of the postoperative inflammation and in patients with complications.
In conclusion, topical Flurbiprofen was comparable to topical Loteprednol and it can be used in reducing the postoperative inflammation in patients who have undergone uncomplicated cat-aract surgeries.
[1]. Harry J, Misson G, Immunity, Inflammation and InfectionClinical Ophthalmic Pathology 2001 New DelhiButterworth Heineman:30-113. [Google Scholar]
[2]. Pavesion CE, Decory HH, The treatment of the ocular inflammatory conditions with Loteprednol etabonateBrit J Ophthalmol 2008 92:455-59. [Google Scholar]
[3]. Hayat AK, Abandan KA, Topical diclofenac versus dexamethasone after strabismus surgeries- A blind randomized clinical trial on the anti-inflammatory effect and the ocular hypertensive responseIndian J Ophthalmol 2007 55:271-75. [Google Scholar]
[4]. Manjoo SR, Suneetha N, Reji KT, Batt RR, Topical diclofenac sodium for the treatment of postoperative inflammationDrugs 2007 67(9):1291-308. [Google Scholar]
[5]. Sihota R, Tondon R, Diseases of the ConjunctivaParson's Diseases of the Eye 2008 20th ed.Noida (India)Elsevier:155-80. [Google Scholar]
[6]. Soon-Phaik C, Seng-Ei T, Meenakshi S, Donald TH, The post-operative inflammation: extracapsular cataract extraction versus phaco-emulsificationJournal of Cataract Surgery 1995 25:1280-85. [Google Scholar]
[7]. Joseph NS, Robyn Ap, Joyce EJ, Comparison of the efficacy and the safety of ketorolac Tromethaminen 0.5% and prednisolone acetate 1% after cataract surgeriesJ Cataract Refract Surg 1999 25:699-704. [Google Scholar]
[8]. Abelson MB, Butrus S, Albert DM, Jakobiec FA, Azar DT, Gragounds Corticosteroids in the Ophthalmic PracticePrinciples and Practice of Ophthalmology 2000 2nd ed.PhiladelphiaWB Saunders Company:258-65.vo0-1 [Google Scholar]
[9]. McGhee Charles NJ, Dean S, Danesh-Meyer H, The locally administered ocular corticosteroids and their benefits and risksDrug Safety 2002 25(1):33-35. [Google Scholar]
[10]. Nussenblatt RB, Whitcup SM, Examination of the patients with uveitisUveitis Fundamentals and Clinical Practice 2004 3rd ed.Mosbay:54-65. [Google Scholar]
[11]. DeLoach LJ, Higgins MS, Caplan AB, Stiff JL, The visual analog scale in the immediate postoperative period: the intrasubject variability and the correlation with a numerical scaleAnesth Analg 1998 86:102-06. [Google Scholar]
[12]. Schimmer BP, Parker KL, Brunton LL, The adrenocorticotrophic hormone-adrenocortical steroids and their synthetic analogs, Inhibition of the synthesis and action of the adrenocortical hormonesGoodman and Gillman's The Pharmacological Basis of therapeutics 2006 11th ed.New DelhiMc Graw Hill:1587-612. [Google Scholar]
[13]. Awan MA, Agarwal PK, Watson DG, McGhee Charles NJ, Dutton GN, Penetration of the topical and the subconjunctival corticosteroids into the human aqueous humor and its therapeutic significanceBrit J Ophthalmol 2009 33:708-13. [Google Scholar]
[14]. Burke A, Smyth E, Fitzgerald GA, Brunton LL, Analgesic antipyretic agentsGoodman and Gillman's Pharmacological Basis of Therapeutics 2006 11th ed.New DelhiMcGrew Hill:671-716. [Google Scholar]
[15]. Solomon KD, Cheetham JK, DeGryse R, Brint SF, Rosenthal A, The topical ketorolac tromethamine 0.5% ophthalmic solution in the ocular inflammation after cataract surgeriesOphthalmology 2001 108(2):331-37. [Google Scholar]
[16]. Miyanaga M, Miyai T, Nejima R, Maruyama Y, Miyata K, Kato S, The effect of the Bromfenac ophthalmic solution on the ocular inflammation following cataract surgeriesActa Ophthalmol 2009 87(3):300-05. [Google Scholar]
[17]. Solomon KD, Turkalj JW, Whiteside SB, Stewart JA, Apple DJ, Topical 0.5% Ketorolac vs 0.03% Flurbiprofen for the inhibition of miosis during cataract surgeriesArch Ophthalmol 1997 115:1119-22. [Google Scholar]
[18]. Reddy MS, Suneetha N, Thomas RK, Battu RR, Topical diclofenac sodium for the treatment of the postoperative inflammation in cataract surgeriesIndian J Ophthalmol 2000 48(3):223-26. [Google Scholar]