Introduction
Urinary Tract Infections (UTIs) are defined as diseases which are caused by a microbial invasion of the genitourinary tract, that extends from the renal cortex of the kidney to the urethral meatus. They represent the most commonly acquired bacterial infections and they account for an estimated 25-40% of the nosocomial infections [1]. The risk of developing urinary tract infections increases significantly with the use of indwelling devices such as catheters and urethral stents or sphincters.
The urinary catheters are tubular latex or silicone devices, which when inserted, may readily acquire biofilms on the inner or outer surfaces. The organisms which commonly contaminate these devices and develop biofilms are Staphylococcus epidermidis, Enterococcus faecalis, Escherichia coli, Proteus mirabilis, Pseudomonas aeruginosa, Klebsiella pneumoniae and other gram-negative organisms [2]. The longer the urinary catheter remains in place, the greater is the tendency of these organisms to develop biofilms, which may result in urinary tract infections.
Biofilms are the microbial communities of the surface-attached cells which are embedded in a self-produced extracellular polymeric matrix [3]. They can cause significant problems in many areas, both in the medical settings (e.g. persistent and recurrent infections, device-related infections) and in the non-medical (industrial) settings (e.g. biofouling in the drinking water distribution systems and in the food processing environments). The biofilms have a major medical significance as they decrease the susceptibility to the antimicrobial agents. Furthermore, the proximity of cells within a biofilm can facilitate a plasmid exchange and hence enhance the spread of antimicrobial resistance [4].
Thus, the present study was done to isolate and to detect the biofilm forming capacity of the uropathogens in patients with catheter associated urinary tract infections.
Materials and Methods
This was a prospective, analytic study which was done for a period of 2 months. A total of 50 urine samples were collected from patients of all the age groups and both the sexes, who had indwelling urinary catheters for at least 2 days, who were suffering from the symptoms of UTIs (fever > 38ºC, urgency, frequency dysuria or suprapubic tenderness). The non-catheterized patients with urinary tract infections were excluded. The study was approved by the institutional Human Ethical Committee (IHEC) and an informed written consent was taken from the patients before the collection of the samples. The samples were collected under complete aseptic conditions with sterile syringes from the distal ends of the urinary catheters and they were transferred to sterile urine containers and transported immediately to the laboratory without any delay.
The urine samples were inoculated onto Blood Agar, MacConkey’s agar and the Cystine Lactose Electrolyte Deficient (CLED) medium with calibrated loops to determine the Colony Forming Units (CFU). The identification of the isolates was done on the basis of the colony morphology, gram staining and the standard biochemical tests.
The Detection of the Biofilm Formation
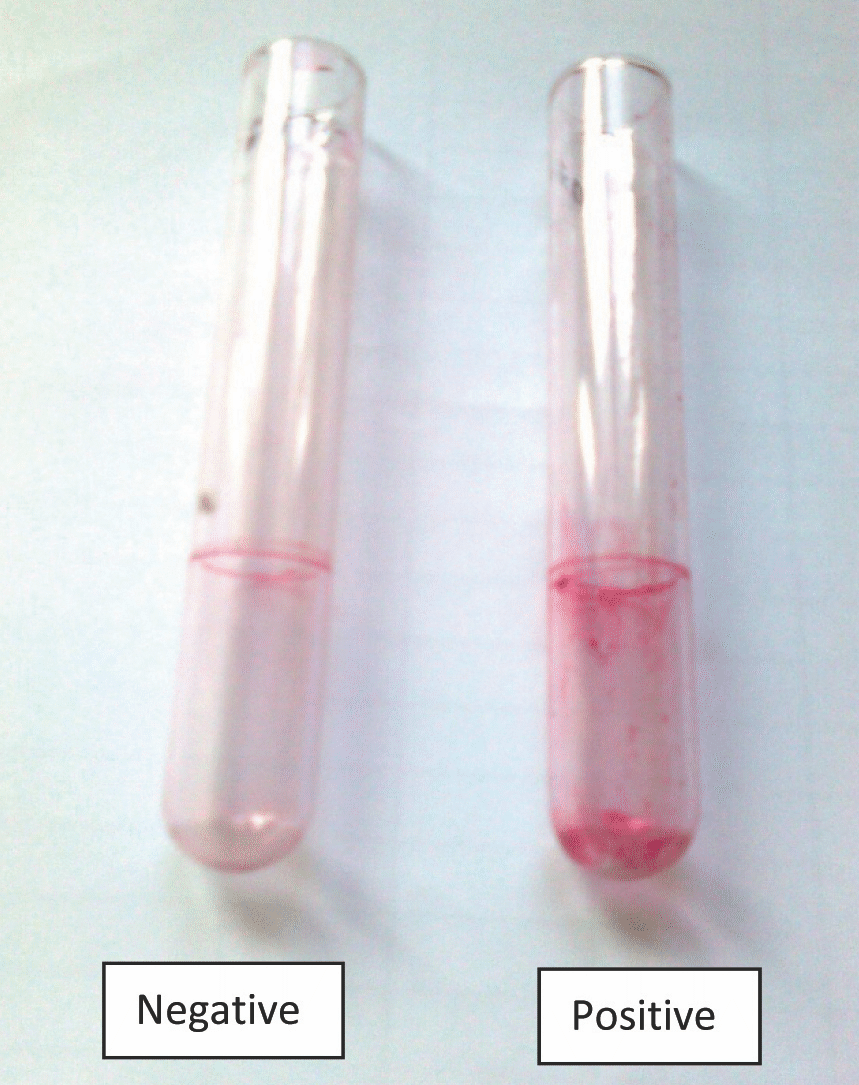
The detection of the biofilms was done by the tube adherence method and the Congo Red agar method. The investigation of the biofilm production was done on the basis of the adherence of the biolfilms to borosilicate test tubes, as was done by Christensen et al. (1982) [5].
The suspensions of the tested strains were incubated in glass tubes which contained Brain Heart Infusion Broth (broth) aerobically at a temperature of 35°C for a period of 2 days. Then, the supernatants were discarded and the glass tubes were stained with a 0.1% Safranin solution, washed with distilled water 3 times and dried. A positive result was defined as the presence of a layer of the stained material which adhered to the inner wall of the tubes. The exclusive observation of a stained ring at the liquid-air interface was considered as negative [Table/Fig-1].
The investigation of the biofilm production by the Congo Red agar method was proposed by Freeman et al. (1989) [6].
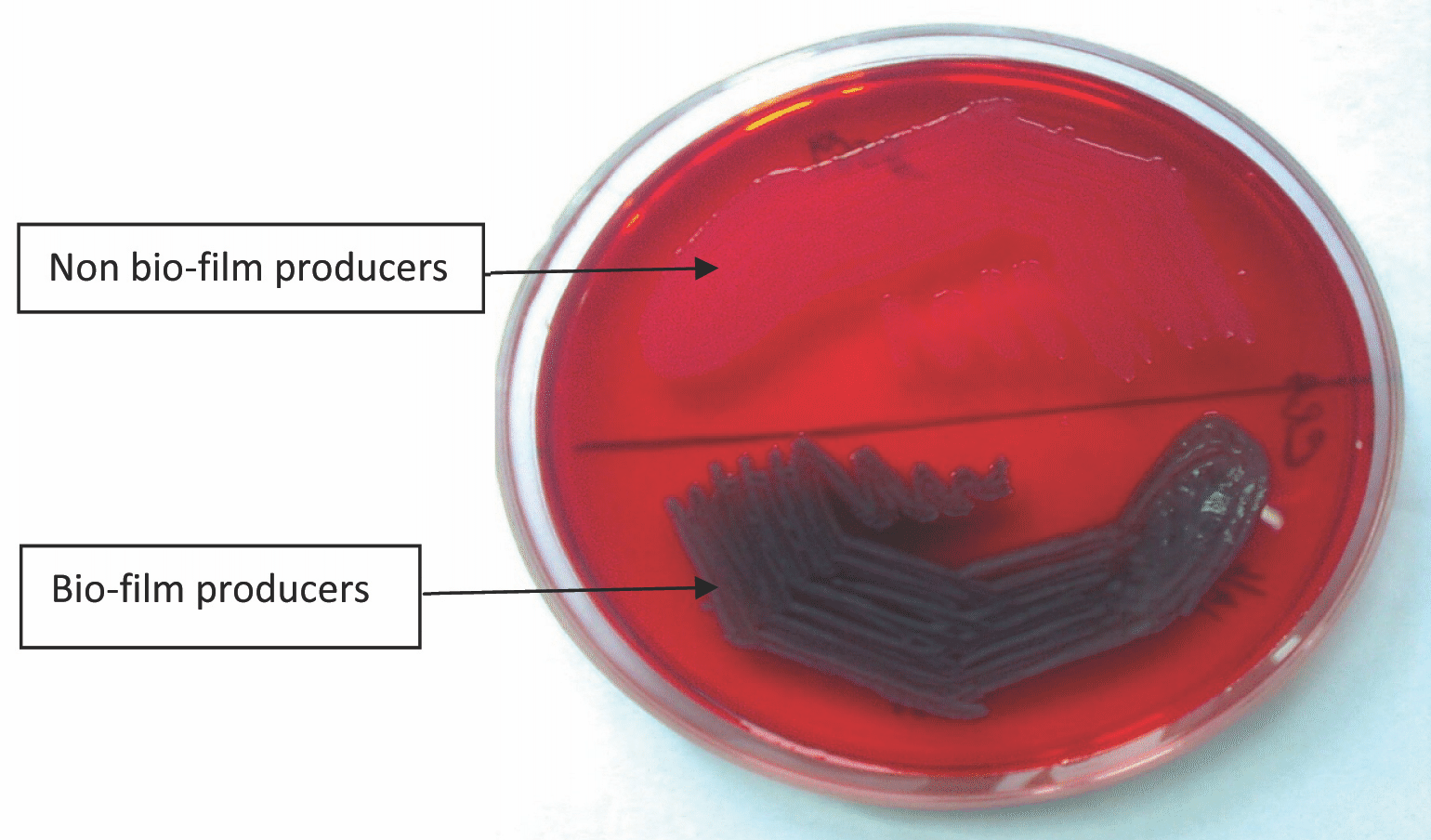
The suspensions of the tested strains were inoculated into tubes which contained a specially prepared solid medium- Brain Heart Infusion broth (BHI) which was supplemented with 5% sucrose and Congo Red. The medium was composed of BHI (37 gms/L), sucrose (50 gms/L), agar no.1 (10 gms/L) and the Congo Red stain (0.8 gms/L). Congo Red was prepared as a concentrated aqueous solution and it was autoclaved at 121°C for 15 minutes, separately from the other medium constituents and it was then added when the agar had cooled to 55°C. The plates were inoculated and incubated aerobically for 24-48 hours at 37°C.
A positive result was indicated by black colonies with a dry crystalline consistency. The weak slime producers usually remained pink, though an occasional darkening at the centres of the colonies was observed [Table/Fig-2]. A darkening of the colonies, with the absence of a dry crystalline colonial morphology, indicated an indeterminate result. The experiment was performed in triplicate and it was repeated 3 times.
Quality Control
The following international reference strains were used as the controls: the biofilm producer, S. epidermidis ATCC 35984 (the positive control) and the non-biofilm producer, S. epidermidis ATCC 12228 ( the negative control).
The biofilm detection by any one of the above methods was considered as positive.
Statistical Analysis
The statistical analysis was done by considering the percentage and the simple ratios.
Results
A total of fifty (50) patients with indwelling urinary catheters, with the symptoms of urinary tract infections, were surveyed in the study period. They were within the age range of 15-90 yrs [Table/Fig-3]. Among the catheterized patients, the age group 61-75 years predominated and it represented 36% of the patients. The rates of the UTIs with respect to the duration of the catheterization has been shown in [Table/Fig-4]. Among the 50 patients, the duration of the catheterization of <4 days was seen in 16(32%) patients, the duration of the catheterization of 4-7 days was seen in 32(64%) patients and the durations of the catheterization of 8-14 days and >14 days were seen in 1(2%) patient each.
Age distribution of patients investigated for UTI
| Range | Number | Percentage |
|---|
| 15-30 | 5 | 10 |
| 31-45 | 10 | 20 |
| 46-60 | 16 | 32 |
| 61-75 | 18 | 36 |
| 76-90 | 1 | 2 |
| Total | 50 | 100 |
Rate of UTI with respect to duration of catheterization
| Duration | Number | Percentage |
|---|
| <4 | 16 | 32 |
| 4-7 | 32 | 64 |
| 8-14 | 1 | 2 |
| >14 | 1 | 2 |
The most common underlying illness was Diabetic mellitus, which was found in 44% of the patients, followed by hypertension in 36% patients, congestive heart failure in 10% patients, pneumonia in 8% patients, bronchial asthma in 4% patients and renal calculi in 4% of the patients [Table/Fig-5]. The number and the percentage of each uropathogen which was isolated from the catheterized urine samples have been shown in [Table/Fig-6]. Among these 50 strains, E.coli was found to be the most frequently isolated pathogen 35(70%), followed by Klebsiella spp 8(16%), Pseudomonas aeruginosa 2(4%), Acinetobacter spp 1(2%), coagulase negative Staphylococci 3(6%) and Enterococci 1(2%).
Distribution of common underlying illness
| Diagnosis | Frequency | Percentage |
|---|
| Diabetes Mellitus | 22 | 44 |
| Hypertension | 18 | 36 |
| Congestive heart failure | 5 | 10 |
| Pneumonia | 4 | 8 |
| Bronchial asthma | 2 | 4 |
| Renal calculi | 2 | 4 |
In the current study, 30(60%) strains were in vitro positive for the biofilm production and 20(40%) were negative for the biofilm production [Table/Fig-6]. The results of the biofilm production by the Congo Red agar method and the tube adherence method showed that there was a complete agreement between the 2 methods in 42 of the total 50 isolates [Table/Fig-7]. A total of 22 isolates were found to be positive by both the methods and 20 strains were found to be negative by both the methods. For the remaining 8 strains, 6 were found to be negative by the Christensen’s method, which were positive on the Congo Red agar and 2 strains were found to be positive by the Christensen’s method, which were negative on the Congo Red agar.
Screening of 50 urinary isolates for biofilm formation by CRA and TA method
| Bacterial Organisms | Slime producers (%) | Non-Slime producers (%) | Total |
|---|
| Escherichia coli | 21(60%) | 14 (40%) | 35 (70%) |
| Klebsiella pneumoniae | 5 (63%) | 3 (37%) | 8 (16%) |
| Pseudomonas aeruginosa | 2 (100%) | – | 2 (4%) |
| Acinetobacter lwoffi | 1 (100%) | – | 1 (2%) |
| Coagulase negative Staphylococci | – | 3 (100%) | 3 (6%) |
| Enterococci spp | 1 (100%) | – | 1(2%) |
| Total | 30 (60%) | 20 (40%) | 50 (100%) |
Results of biofilm production by Congo red and Christensen method
| Total No of Isolates | Congo red method | Christensen method |
|---|
| 22 | + | + |
| 20 | − | − |
| 6 | + | − |
| 2 | − | + |
Discussion
Urinary Tract Infections (UTIs) are one of the most common bacterial infections in humans. A patient was said to be suffering from a Catheter-Associated Urinary Tract Infection (CAUTI) if he was catheterized and if he developed one or more of the following conditions, that is fever (temp ≥ 38°C) without any other known cause, urgency or suprapubic tenderness, with the urine culture showing more than 10 [5]colony-forming units or more per ml of urine, with not more than 2 types of organisms. A CAUTI was also considered when the urine showed pyuria (more than 10 leukocytes per ml of the urine), when more than three WBCs were seen per high-power field in the centrifuged urine, when the organisms were seen on gram staining or when the clinician strongly suspected UTIs to start with antibiotics.
In this study, the UTIs were more common in the elderly patients (who were aged > 60 years). Similar findings had been reported among the non institutionalized elderly populations, in which genitourinary infections were the second most common form of the infections, accounting for nearly 25% of all the identified infections [7]. This suggested that the elders who resided in the long-term care facilities had a high prevalence of the chronic genitourinary symptoms and bacteriuria, and that they were at a risk for the urinary catheter-associated infections for a definitive diagnosis of UTIs.
For either the short- or long-term catheters, the infection rate was about 5% per day [8]. The detection of bacteriuria within 1 week of the catheterization in this study may be related to the inadequate precautions which were taken. The catheter associated urinary tract infections can sometimes be prevented in the patients who are catheterized for < 2 weeks with the use of a sterile closed collecting system, by paying attention to the aseptic techniques during the insertion and the care of the catheters, and by taking measures to minimize the cross-infections [9].
Diabetes (44%) was the most common factor which was associated with the UTIs in our study, which correlated with the findings of other studies from south India [10]. Diabetes mellitus produces a number of long-term effects on the genitourinary system. Diabetic nephropathy is one of the many factors that make these patients more susceptible to UTIs than the non diabetics. The reduced immunity in Diabetes also contributes to the increased risk for acquiring UTIs.
The present study showed that among these 50 strains, E.coli was the most frequently isolated pathogen 35(70%), followed by Klebsiella spp 8(16%), Pseudomonas aeruginosa 2(4%), Acinetobacter spp 1(2%), coagulase negative staphylococci 3(6%) and Enterococci 1(2%), which was similar to the findings of Hassin [11] who reported that in their study, E.coli (74%) was the predominant organism, followed by Klebsiella spp 17.7% and Pseudomonas spp 2.5%. Ronald, in his study, found that E. coli remained the predominant uropathogen (80%) in the community acquired infections, followed by S. saprophyticus (10-15%), Klebsiella, Enterobacter and Proteus spp [12].
Escherichia coli is responsible for more than 80% of all the UTIs and it causes both symptomatic UTIs and Asymptomatic Bacteriuria (ABU) [13, 14]. The ability of the Uropathogenic E. coli (UPEC) to cause symptomatic UTIs is associated with the expression of a variety of virulence factors, which include adhesins (e.g., type 1 and P fimbriae) and toxins (e.g., haemolysin) [14,15].
A biofilm is a thin layer of micro-organisms that adhere to the surface of an organic or inorganic structure, together with their secreted polymers. Biofilms are the predominant phenotype of nearly all the bacteria in their natural habitats, whether they are pathogenic or environmental.
In the current study, 30 (60%) strains were positive in vitro for the biofilm production. A similar study showed a 73% biofilm production by the uropathogens from the UTIs [16]. A significant production of biofilms was seen in 21 (60%) isolates of E. coli, whereas Sharma et al., reported a similar rate of the biofilm production (67.5%) in E.coli. The 70.3% biofilm production was more in the patients with catheters [17]. Another study which was done by Ponnusamy et al., [18] showed that among 100 (60.2%) E. coli strains, 72 strains displayed a biofilm positive phenotype.
The biofilms which produce E. coli are recalcitrant to the immune factors and the antibiotic therapy and they are often responsible for the chronic UTIs. Usually, the floating cells are completely eradicated at the antibiotic levels which are predicted by the laboratory Minimum Inhibitory Concentration (MIC) studies. However, more than 100 times the MICs of the antibiotics are required to eradicate the cells within the biofilm. Therefore, an effectual control will need an intensive effort to build up newer therapeutic agents that aim at preventing the formation of biofilms or encouraging the biofilm detachment.
In the present study, 2 different methods were carried out for the detection of the slime production. The detection of the biofilm production by the Congo Red agar method and the tube adherence method were (56%) and (44%) respectively. Both the methods which have been described here were based on the enhancement of the exopolysaccharide production by using the enriched medium, TSB, in the Christensen method [5], while the Congo Red agar method also required the use of a highly nutritious medium- in this case, the brain heart infusion broth with a 5% sucrose supplementation. Pfaller et al., [19] in their studies, showed that the Congo Red method was rapid, more sensitive, and reproducible and that it had the advantage of the colonies remaining viable on the medium.
The microbial biofilms have been associated with a variety of persistent infections which respond poorly to the conventional antibiotic therapy. This also helps in the spread of antibiotic resistant traits in the nosocomial pathogens by increasing the mutation rates and by the exchange of the genes which are responsible for the antibiotic resistance. The antibiotic therapy against the device associated biofilm organisms often fails without the removal of the infected implant. The physiological heterogeneity is another important characteristic which is observed in the biofilm bacteria. This phenomenon affects the rate of growth and the metabolism of the bacteria and it is reflected by the interbacterial quorum signals, the accumulation of the toxic products and the change in the local micro environment. These so called persister cells are not resistant to the antibiotics per se, but they become resistant when they are associated with the biofilm [20].
Conclusion
To conclude, there was significant bacteriuria in all the symptomatic catheterized patients and E.coli was the most frequent isolate in the urinary tract infections in the catheterized patients. The Diabetes and urogenital instrumentation were the major risk factors for the UTIs. The microbial biofilms may pose a public health problem for the persons who require indwelling medical devices, as the microorganisms in the biofilms are difficult to treat with antimicrobial agents. Also, there is a need to establish standard guidelines on the care of catheters for all the units in the hospital, with a view to prevent the nosocomial infections which are associated with the devices in the patients.
Acknowledgement
We would like to thank the ICMR for sponsoring this Short Term Studentship (STS) project.
[1]. Bagshaw SM, Laupland KB, The epidemiology of the intensive care unit acquired urinary tract infectionsCurr Opin Infect Dis 2006 19:67-71. [Google Scholar]
[2]. Stickler DJ, The bacterial biofilms and the encrustation of the urethral cathetersBiofouling 1996 94:293-305. [Google Scholar]
[3]. Donlan RM, Costerton JW, Biofilms: the survival mechanisms of the clinically relevant microorganisms.Clin Microbiol Rev 2002 15:167-93. [Google Scholar]
[4]. Watnick P, Kotler R, A biofilm, a city of microbesJ. Bacteriol 2000 182:2675-79. [Google Scholar]
[5]. Christensen GD, Simpson WA, Bismo AL, Beachery EH, The adherence of the slime-producing strains of Staphylococcus epidermidis to smooth surfacesInfect immune 1982 37:318-26. [Google Scholar]
[6]. Freeman DJ, Falkiner FR, Keane CT, A new method for the detection of the slime production by the coagulase negative StaphylococciJ Clin Pathol 1989 42:872-74. [Google Scholar]
[7]. Madigan E, Neff D, Care of the patients with long-term indwelling urinary cathetersOnline J Issues Nurs 2003 8:7 [Google Scholar]
[8]. Nicolle LE, The catheter-related urinary tract infectionsDrugs Aging 2005 22:627-39. [Google Scholar]
[9]. Braunwald E, Fauci AS, Kasper DL, Hauser SL, Longo DL, Jameson JL, Harrison’s Principles of Internal Medicine 2001 15th ed.New York, USAMcGraw-Hill:1620-25. [Google Scholar]
[10]. Eshwarappa M, Dosegowda R, Vrithmani Aprameya I, Khan MW, Shiva Kumar P, Kempegowda P, The clinico-microbiological profile of the urinary tract infections in south IndiaIndian J Nephrol 2011 21:30-36. [Google Scholar]
[11]. Hassin SKR, Studies on urinary tract infectionsBangladesh Medical Journal 1991 20:29-32. [Google Scholar]
[12]. Ronald A, The etiology of urinary tract infections: the traditional and the emerging pathogensDis Mon 2003 49:71-82. [Google Scholar]
[13]. Hedlund M, Duan RD, Nilsson A, Svensson M, Karpman D, Svanborg C, The fimbriae, the transmembrane signaling, and the cell activationJ Infect Dis 2001 183:S47-S50. [Google Scholar]
[14]. Svanborg C, Godaly G, The bacterial virulence in urinary tract infectionsInfect. Dis. Clin. North Am. 1997 11:513-29. [Google Scholar]
[15]. Klemm P, Schembri MA, The bacterial adhesins: their functions and structuresInt. J. Med. Microbiol 2000 290:27-35. [Google Scholar]
[16]. Reid G, Charbonneau-Smith R, Lam D, Kang YS, Lacerte M, Hayes KC, The bacterial biofilm formation in the urinary bladder of spinal cord injured patientsParaplegia 1992 30:711-17. [Google Scholar]
[17]. Sharma M, Aparna Yadav S, Chaudhary U, The biofilm production in the uropathogenic Escherichia coliIndian J Pathol Microbiol 2009 52:294 [Google Scholar]
[18]. Ponnusamy P, Natarajan V, Sevanan M, The in vitro biofilm formation by the uropathogenic Escherichia coli and their antimicrobial susceptibility patternsAsian Pac J Trop Med. 2012 5(3):210-13. [Google Scholar]
[19]. Pfaller MA, Davenport D, Bale M, Barret M, Koontz F, Massanari R, The development of the quantitative micro-test for detecting the slime production by the coagulase negative StaphylococciEur J Clin Microbiol Infect Dis 1988 7:30-33. [Google Scholar]
[20]. Simon AL, Robertson GT, Bacterial and fungal biofilm infectionsAnnual Review of Medicine 2008 59:415-28. [Google Scholar]