Introduction
The Shigella species cause an invasive gastroenteritis and are also causes of traveller’s diarrhoea. In the travellers who go to the developing countries, diarrhoea is the most frequent symptom which is seen, when they are exposed to this infectious agent. Shigellosis is very common in children and it is one of the leading causes of morbidity and mortality in this group in the developing countries. In the endemic areas, the children who are less than 5 years of age have the highest rate of incidence. After the age of 5 years, the incidence declines, thus suggesting that a protective immunity develops after the exposure and that it is serotype specific [1,2].
Any member of the genus, Shigella, namely S. dysenteriae, S. flexneri, S. sonnei and S.boydii, can produce Shigellosis. Among the pathogenic Shigella subgroups, S. flexneri is the most commonly detected strain in the infectious processes in the Asian countries [2].
Though the control of the severe disease, shortening the duration of the fever, diarrhoea and toxaemia, in reducing the risk of lethal complications, reducing the excretion of the pathogen in the stools significantly and reducing the spread of the infection warrants the appropriate chemotherapy, the unfortunate increase in the anti-microbial resistance across the globe has been an area for concern. Conventionally, the fluoroquinolones have been the mainstay of the treatment for Shigellosis. With the emergence of fluoroquinolone resistance among the Shigellae [2–7], the choice of the antibiotics has been reduced further.
The present study reflects upon the changing antibiotic resistance pattern in the Shigella spp. in our region.
Materials and methods
This study was carried out in the Department of Microbiology, JIPMER, Puducherry. A total of 2658 stool samples from patients with diarrhoea were received between 2008 and 2010. The stool specimens which were received were inoculated onto Mac Conkey’s agar, deoxycholate citrate agar media, xylose lysine deoxycholate agar media and selenite-F enrichment broth, and these media were incubated at 37ºC overnight. Subcultures from the selenite-F broth was done after 18 hours onto Mac Conkey’s agar, deoxycholate citrate agar media and xylose lysine deoxycholate agar media [10]. The Shigella strains were identified, based on their cultural characteristics and various biochemical tests [10] and they were confirmed by doing the slide agglutination test by using the specific antisera (Denka-Seiken, Tokyo, Japan). Disc diffusion testing was performed by using the Kirby Bauer method as per the Clinical Laboratory Standards Institute guidelines [11] against ampicillin (A)10μg, ceftriaxone (Ci)30μg, ciprofloxacin (Cf)5μg, nalidixic acid (Na)30μg, furoxone (Fx)300μg, chloramphenicol(C) 30μg and cotrimoxazole(Co) 25μg.
The Minimum Inhibitory Concentrations (MICs) of ceftriaxone and ciprofloxacin were determined by the agar dilution method and the E-test. For the agar dilution method, the ceftriaxone-Sodium salt (Himedia, Mumbai, India ) and the ciprofloxacin hydrochloride powder (Himedia, Mumbai, India) were dissolved respectively in sterile distilled water as was described by the manufacturer. The drugs, after their reconstitution, were stored at 4ºC and were used within 2 days of their reconstitution. Different dilutions of the antibiotics were used as per the recommendations [12,13]. ATCC Escherichia coli 25922 was inoculated on each plate as the growth control. The E-test was performed as per the manufacturers’ instructions (Biomeriuex, India). The double disk synergy test was carried out on the ceftriaxone resistant isolates [13].
Results
A total of 74 (2.78%) Shigella spp. were identified from the 2658 stool samples which were received in the Stool Section, Department of Microbiology, JIPMER, Puducherry, in the time period from January 2008 to December 2010 [Table/Fig-1]. These were isolated from patients whose ages ranged from 0 to 45 years.
The distribution of the Shigella spp isolated from children and adults
| Shigella spp. | Total Number (n=74) | Number of isolates in different age groups | Clinical manifestations associated | Antimicrobial resistance pattern in 2008 | Antimicrobial resistance pattern in 2009 | Antimicrobial resistance pattern in 2010 |
|---|
| 0 to ≤5 years | ≤15 years | ≥15 years |
|---|
| S. dysenteriae | 2 (2.70%) | 2 (100%) | 0 | 0 | Dysentery=1 AGE=1 | A=Co | A=Co=Na=Cf=Fx=C | Nil |
| S. flexneri | 67 (90.54%) | 37 (55.22%) | 12 (17.91%) | 18 (26.86%) | Dysentery=42 AGE=18 Chronic diarrhoea=4 Shigella encephalopathy=3 | A=Co>Na>Cf>C>Fx | A=Co=Na=Cf>C>Fx | A=Co>Cf>Fx=C>Ci Na was not tested |
| S. boydii | 1 (1.35%) | 1(100%) | 0 | 0 | AGE=1 | A | A=Na=C=Fx=Co | Nil |
| S. sonnei | 4 (5.40%) | 3(75%) | 1(25) | 0 | Dysentery=3 AGE=1 | Co>A=Na=C>Cf=Fx | Nil | A=Cf=Fx=Co |
Abbreviations used: A-Ampicillin, Na-Nalidixic acid, Cf-Ciprofloxacin, C-Chloramphenicol, Ci-Ceftriaxone, Fx-Furazolidone, AGE-Acute gastroenteritis.
Of these, 43 (58.108%) strains were isolated from children of 0 to ≤5 years, 13(17.56%) were isolated from children who were >5 years but ≤ 15 years of age and the rest of the 18 (24.32%) were isolated from adult patients [Table/Fig-2]. Only S.flexneri could be isolated from patients who were >15 years of age. The rest of the strains were found to be associated with the paediatric age group. The predominant manifestation which was common to all the strains was dysentery, except S.boydii, of which only one strain was isolated.
Comparison of isolation of Shigella spp.from different places
| Isolates from different places | Peru (2008) ref 5 | Kolkata (2003) ref 3 | Bangalore (2009)ref 4 | PGIMER (2005) ref6 | JIPMER (present study) |
|---|
| S.dysenteriae | 2.4% | 9.37% | 3.7% | 23.4% | 2.70% |
| S.flexneri | 67.1% | 60.4% | 64.9% | 61.7% | 90.54% |
| S.boydii | 11.4% | 6.4% | 8.2% | 7.45% | 1.35% |
| S.sonnei | 11.8% | 23.8% | 21.6% | 7.45% | 5.40% |
| Total | 7.2% | - | 4.6% | - | 2.78% |
Out of the 74 isolates, 35 (50%) strains were resistant to ciprofloxacin, with an MIC of 16μg/ml. 2 (3%) strains were found to be resistant to ceftriaxone, with MICs of > 256µg/ml. These 2 ceftriaxone resistant isolates were identified as S. flexneri: one of these strains was isolated from a child who was <5 years of age and the other was isolated from an adult of 18 years of age. These 2 strains of S. flexneri were further confirmed to be ESBL producers phenotypically by the Double Disk Synergy Test by using a ceftazidime (Ca) 30μg and ceftazidime 30μg plus clavulanic acid 10μg (CaC) disk (Himedia, Mumbai, India).
Discussion
All the species of Shigella cause an acute bloody diarrhoea by invading and causing a patchy destruction of the colonic epithelium. This leads to the formation of micro-ulcers and inflammatory exudates, and causes inflammatory cells (polymorphonuclear leucocytes and PMNs) and blood to appear in the stool. In the present study, the predominant outcome of the infection with any of the strains was dysentery, followed by acute gastroenteritis [Table/Fig-1]. This was in contrast to the clinical presentation in Peru [5], where acute gastroenteritis (AGE) was the commonest manifestation of Shigellosis, which was noted in 79.8% of the cases. 73.4% cases had fever associated with them, 30.8% had dysentery and 3.2% were asymptomatic. In the present study, AGE was observed in 28.37% cases, while dysentery was the commonest manifestation in 62.16% cases, chronic diarrhoea was noted in 5.45% cases and 4.05% cases showed Shigella encephalopathy. It was noted that only S.flexneri could be isolated from the paediatric as well as the adult age groups, whereas all the other strains remained confined to the paediatric age group. The manifestation of S.flexneri was more variable as compared to the other strains. The infection with any of the members of the genus, Shigella can result in persistent and chronic diarrhoea, especially in children with a protein energy malnutrition. We had 4 such children with chronic diarrhoea, from whom S.flexneri was isolated. Shigella encephalopathy was observed in three cases of infection with S.flexneri (TableI). Shigella encephalopathy is a neurologic manifestation which arises due to metabolic derangements such as electrolyte imbalances or hypoglycaemia and it is mostly associated with S.flexneri. Clinically, an altered consciousness with a bizarre posturing and unresponsiveness are noted [17].
Resistance pattern in S.flexneri over the years.
Y–axis: percentage of strains resistant, X-axis: antibiotics tested; A-ampicillin, Ci-ceftriaxone, Cf-ciprofloxacin, Fx-furoxone, Co-cotrimoxazole
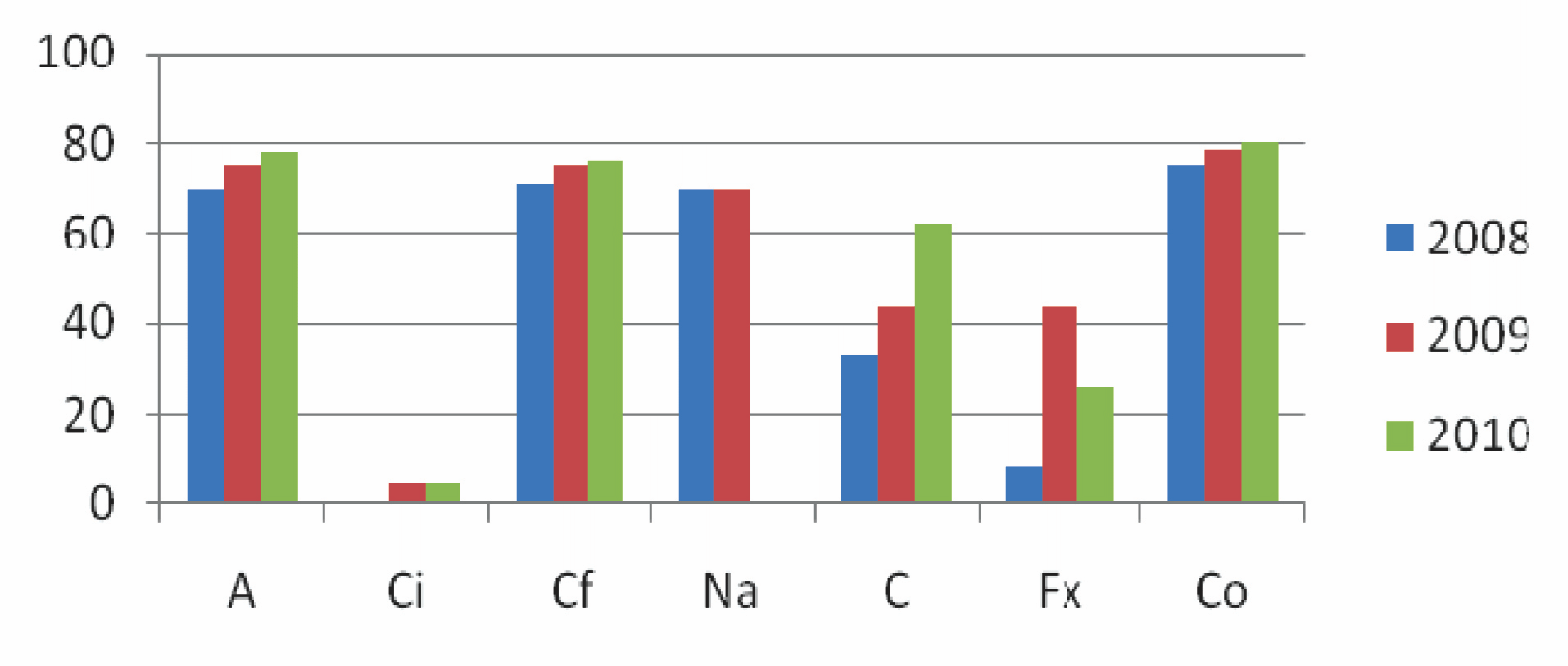
Although, the cyclical changes in the serotypes is a known fact, the factors which govern this phenomenon are exactly not known. The most common (90.54%) circulating serotype which was determined in our region was S. flexneri, followed by S.sonnei, S.dysenteriae and S.boydii. This trend of the strain variation pattern was probably similar to that of Kolkata [Table/Fig-2].
The commonest age group which was involved was clearly the children of < 5 years of age with 43 (58.108%) strains of Shigella being isolated from their stool samples [Table/Fig-2]. Children were more often involved than the adults (78.72% vs 21.27%). This finding was similar to that of a study which was done in Peru [5] where children were involved more than adults (80% vs 20%). It also showed similarity with a report from Chandigarh [18] in which children were involved more commonly (61.3%) than adults (29%) and it was in contrast to an earlier report from Bangalore [4], where children were less involved than adults (41% vs 58.2%).
The choice of the antibiotics for treating any infectious disease in children rests on a number of factors, especially the route of administration; among which the oral route is preferred. Unfortunately, the Shigella strains which were isolated from children in our centre were resistant to ampicillin and cotrimoxazole, in nearly >70% of all the cases, thus limiting the choice of the orally administrable antibiotics. A progressive trend of the resistance pattern was observed, with nearly >90% of the strains being resistant to more than two antibiotics by the turn of 2010. The most dramatic and alarming increase in the drug resistance was noted in S.flexneri. The strains (~80%) of S.flexneri which were isolated in 2010 were resistant to more than two groups of antibiotics as compared to those which were isolated in 2009 (~75%) and 2008 (~70%) respectively [Table/Fig-3]. Ciprofloxacin is very easily available and it is used most commonly, which has contributed to the resistance to this antibiotic. In the present study, the MIC values which were obtained were similar to that from earlier studies which were done in India [18]. More so, injectables like the third generation cephalosporins have not been spared. The high MIC value of ceftriaxone which was observed in some of our strains was an alarming finding. The emerging pattern of resistance in our centre was ampicillin>cotrimoxazole>nalidixicacid>ciprofloxacin>furoxone=chloramphenicol>> ceftriaxone, in the order of the decreasing resistance.
Ciprofloxacin has been recommended by the WHO [1] as the drug of choice for all the patients with Shigellosis, irrespective of their ages. Apart from ciprofloxacin, some other fluoroquinolones, pivmecillinam (amdinocillin, pivoxil), ceftriaxone and azithromycin are currently the only anti-microbial s that are usually effective for the treatment of the multi-resistant strains of Shigella in all the age groups. The use of these alternative drugs is however, currently limited by their high costs (pivmecillinam, azithromycin), the rapid development of resistance (azithromycin), their formulation (injectable for ceftriaxone, four times a day for pivmecillinam), and the limited data on their efficacy (azithromycin).
The emergence of the ciprofloxacin resistance [18] has been widespread and this has shifted the focus to other alternatives, namely the cephalosporins. Unfortunately, the recent reports of cephalosporin resistance [14–16] has now really limited the options for the therapy.
In the past 2 decades, both the isolation frequencies and the types of the extended spectrum beta-lactamases (ESBLs), have gradually increased. ESBLs have been noted in Shigella as well. Molecular evidences have confirmed that the ESBL genes are plasmid borne. The Shigella species usually harbour a heterogeneous population of plasmids which range in numbers from 2 to as many as 10 [16]. The ability of these organisms to acquire the resistance genes which are located on the mobile elements, the continuous selective pressure which results from the high levels of the antibiotic consumption and the presence of the poor quality unlicensed antibacterial agents in some areas, enhances the likelihood of the development of resistance [2,7–9,13,14]. The molecular characterization of the strains is underway, which will throw more light on the details of the drug resistance.
The emerging drug resistance pattern has created a very dismal picture, with limited therapeutic options being available to combat it. The emergence of such strains can become a serious threat to the public health, as their spread among a population in which diarrhoeal disease is one of the major causes of childhood morbidity and mortality, calls for a greater attention to the appropriate use of the antibiotics, the establishment of hygienic measures to prevent or to decrease the transmission of this disease, and the development of new effective drugs that can be safely used in children. There is an impending need of updating the guidelines for the treatment of Shigellosis in the developing countries. In this context, the continuous assessment of the anti-microbial susceptibility patterns and stringent methods like strict adherence to the anti-microbial policies may place a check on this growing menace.
Key messages
This study reflected and highlighted the increasing anti-microbial resistance in the Shigella strains which were isolated from our patients. Also, this brought into consideration, the emergence of the resistance in a number of anti-microbial agents, which included the third generation cephalosporins. This data may have a considerable impact on the treatment of Shigellosis and it highlights the role of the continuing surveillance for the drug resistance.
[1]. The WHO, Guidelines for the Control of Shigellosis, including the epidemics which were caused by Shigella dysenteriae type 1 2005 GenevaWorld Health Organization [Google Scholar]
[2]. Seidlein LV, Kim DR, Ali M, Lee H, Wang XY, Thiem VD, A multi-centre study on Shigella diarrhoea in six Asian countries: the disease burden, clinical manifestations, and the microbiologyPLoS Med 2006 3:e353 [Google Scholar]
[3]. Niyogi SK, Pazhani GP, The multi-resistant Shigella species which were isolated from the childhood diarrhea cases in Kolkata, IndiaJpn J Infect Dis 2003 56:33-34. [Google Scholar]
[4]. Srinivasan H, Baijayanthi M, Raksha Y, The magnitude of the drug resistant ShigellosisIndian J Med Microbiol 2009 27:358-60. [Google Scholar]
[5]. Kosek M, Yori PP, Pan WK, Olortegui MP, Gilman RH, Perez J, The epidemiology of the highly endemic, multiple antibiotic-resistant Shigellosis in children in the Peruvian AmazonPediatr 2008 122:e541-e549. [Google Scholar]
[6]. Taneja N, Lyngdoh V, Vermani A, Mohan B, Rao P, Singh M, The re-emergence of multi-drug resistant strains of Shigella dysenteriae with an added resistance to ciprofloxacin in north India and their plasmid profilesIndian J Med Res 2005 122:348-54. [Google Scholar]
[7]. Pazhani GP, Niyogi SK, Singh AK, Sen B, Taneja N, Kundu M, The molecular characterization of the multi-drug resistant Shigella spp which were isolated from the epidemic and the endemic cases of Shigellosis in IndiaJ Med Microbiol 2008 57:856-63. [Google Scholar]
[8]. Rowe-Magnus DA, Guerout A-M, Mazel D, Bacterial resistance evolution by the recruitment of super-integron gene cassettesMol Microbiol 2002 43:1657-69. [Google Scholar]
[9]. White PA, McIver CJ, Rawlinson WD, Integrons and gene cassettes in the EnterobacteriaceaeAntimicrob Agents Chemother 2001 45:2658-61. [Google Scholar]
[10]. Duguid JP, JG Collee, Fraser AG, Marmion BP, Simmons A, ShigellaMackie and McCartney. Practical Medical Microbiology 1999 14th EditionNew YorkChurchill Livingstone:405-12. [Google Scholar]
[11]. The Clinical and Laboratory Standards InstitutePerformance Standards for Anti-microbial Susceptibility Testing, twenty first informational supplement, M100-S21 2011 Wayne, PAClinical and Laboratory Standards Institute [Google Scholar]
[12]. Jorgensen JH, Turnidge JD, Murray P.R., Baron E.J., Jorgensen J.H., Pfaller M.A., Yolken R.H., The susceptibility test methods: The dilution and the disk diffusion methodsManual of Clinical Microbiology 2007 9th ed.Washington, D.C.American Society for Microbiology Press:1108-27.Chapter 70: [Google Scholar]
[13]. Jarlier V, Nicolas M-H, Fournier G, Philippon A, The extended broad-spectrum β-lactamases confer a transferable resistance to the newer β-lactam agents in the Enterobacteriaceae: the hospital prevalence and the susceptibility patternsRev Infect Dis. 1988 10:867-78. [Google Scholar]
[14]. Radice M, Gonzalez C, Power P, Vidal MDC, Gutkind G, Third-generation cephalosporin resistance in Shigella sonnei in ArgentinaEmerg Infect Dis. 2001 7:1-2. [Google Scholar]
[15]. Vinh H, Baker S, Campbell J, The rapid emergence of third generation cephalosporin resistant Shigella spp. in southern VietnamJ Med Microbiol 2009 58:281-83. [Google Scholar]
[16]. Mandal J, Mondal N, Mahadevan S, Parija SC, The emergence of resistance to the third-generation cephalosporins in Shigella- A case reportJ Trop Paediatr 2010 56:278-9.Epub 2009 Dec 2 [Google Scholar]
[17]. Keusch GT, Gorbach SL, Bartlett JG, Blacklow NR, ShigellosisInfectious diseases 2004 third editionLippincott Williams and Wilkins:604-07. [Google Scholar]
[18]. Taneja N, The changing epidemiology of Shigellosis and the emergence of ciprofloxacin resistant Shigellae in IndiaJ Clin Microbiol 2007 45:678-79. [Google Scholar]