Background
This study determines the protein carbonyls which cause cellular damage and glutathione, glutathione peroxidase, glutathione reductase act as antioxidants.
Materials and Methods
This study was carried out in different categories of pulmonary and extra pulmonary tuberculosis cases of newly sputum culture positive diagnosed pulmonary categorie I (n=100), extra pulmonary patients categorie (n=35) before and after the DOTS treatment of 6 months, categorie II (n=100), categorie III (n=100) and in normal control subjects (n=100).
Results
The serum protein carbonyl levels were significantly increased in the pulmonary and extra pulmonary tuberculosis patients. The activities of blood glutathione, glutathione peroxidase, and glutathione reductase were found to be significantly decreased in subjects of all the categories of pulmonary and extra pulmonary tuberculosis. A negative correlation between the carbonyl protein content and glutathione, glutathione peroxidase, and glutathione reductase was seen in pulmonary tuberculosis, p<0.001.
Conclusion
Increased antioxidant defense mechanism due to increase oxidative stress in tuberculosis. The changes were reversed after 6 months of antitubercular treatment in patients with a good recovery, but the increase in the oxidative stress was not completely reversed.
Introduction
Tuberculosis is a chronic granulomatous disease which is caused by Koch’s bacilli, which is called Mycobacterium tuberculosis. The lung is the portal entry of Mycobacterium tuberculosis and it provides a congenial environment for this slowly replicating pathogen. The infection is established in the alveolar macrophages of the distal alveoli [1]. Proteins constitute the major ‘working force’ for all forms of biological work. The Reactive Oxygen and Nitrogen Species (ROS and RNS) are formed in higher fluxes under pathological conditions. They cause cellular damage due to the oxidation of amino acid residues on proteins, forming protein carbonyls [2] (aldehydes, ketones e.g. Lys, Arg, Pro, Thr). Oxidative stress also increases the protein oxidation [3]. The modification of proteins is initiated mainly by reactions with OH; however, the course of the oxidation process is determined by the availability of O2 and O2- or its protonated form (HO2). Collectively, these reactive oxygen species can lead to oxidation of the amino residue side chains, formation of protein-protein cross-linkages and oxidation of the protein backbone, resulting in protein fragmentation, that transition metal ions substitute for OH and O2- [4].
Glutathione, which is involved in the transport of amino acids, acts as a coenzyme for enzymes and it protects against oxygen radicals and toxic compounds [5]. Se- dependent GSH peroxidase is capable of utilizing hydrogen peroxide (H2O2) and a variety of organic hydroperoxides as substrates. The Se-independent activity is attributed to the isoenzymes of the GSH S-transferases, particularly GSH S-transferass B which acts on the organic hydroperoxides but not on hydrogen peroxide. GSH is the electron donor which is of physiological importance for both types of GSH peroxidases [6]. All the four Se-containing enzymes with the GSH peroxidase activity which are characterized to date are encoded by nuclear DNA and they have been mapped to human chromosome 21, 3 and X for C-GSH; to chromosome 19 for PH-GSH peroxidase; to chromosome 5 for P-GSH; and to chromosome 14 for GI-GSH peroxidase. The cDNA sequence of all the four GSH peroxidase isoenzymes contains a UGA codon coding for selenocysteine [7].
The oxidative changes to proteins which are due to carbonyl proteins, can lead to diverse functional consequences such as inhibition of the enzymatic activities, proteolysis and an altered immunogenicity. The activated macrophages may release lytic enzymes and RNI. It also secretes cytokines like interleukin 1 (IL-l), Tumour Necrosis Factor (TNF), and gamma interferon (IFN-) [8]. Because of the low pH and the anoxic environment, Mycobacterium tuberculosis cannot develop in macrophages and it persists for extended periods. We planned to estimate serum protein carbonyl and antioxidant glutathione to know their relationship in newly diagnosed cases of pulmonary tuberculosis and extra pulmonary tuberculosis also in different categories of tuberculosis before stating the treatment and after six months of treatment.
Materials and Methods
This study were undertaken in age group of 16 to 60 years, which was divided into groups on the basis of the diagnosis of different categories of pulmonary with newly sputum culture positive (50 male and 50 female) and extra pulmonary (19 males and 16 females) tuberculosis and normal subjects of 100(50 male and 50 female). Categorie I (n=100), extra pulmonary categorie I (n=35) before and after the DOTS treatment of 6 months, before starting the treatment for categorie II (n=100) and before starting the treatment for categorie III (n=100) were selected from the TB and Chest Diseases Department, OPD and IPD of Sir J.J. Group of Hospitals, G.T. Hospital, Municipal corporation group of TB Hospitals, Sewri, Mumbai, Maharashtra, India. The patients with chronic diseases, hepatitis, diabetes, renal impairment, cardiovascular comorbidities, neurological psychiatric disorders, the Human Immuno-deficiency Virus (HIV) infection and various malignancies and heavy smokers, alcoholics and tobacco chewers were excluded from the study. Approval for the study was taken from the medical ethical committee of the institute [No-IEC/Pharm/379/07, dated 30/8/2007] and an informed consent was taken from all the groups.
Venous blood samples were collected in plain and EDTA vacutainers by following aseptic precautions. The plain blood samples, after 2hrs of collection, were centrifuged at 3000rpm for 5min. The sera were separated and they were collected in sterile Eppendroff tubes. Those with no signs of haemolysis were used for the analysis of the blood protein carbonyl content [9] and the glutathione concentration [10], by using a Randox kit for glutathione peroxidase (GSH-Px) [11], and glutathione reductase (GSH-Rx) [12] was estimated by by using a UV-spectrophotometer (Jasco-V670), and an auto analyzer (Olympus AU-400). The statistical analysis was done by using software Mini tab 16 of the Student’s t test.
Observation and Results
As Shown [Table/Fig-1],[Table/Fig-2],[Table/Fig-3], [Table/Fig-4] and [Table/Fig-5].
Protein carbonyl, Total blood glutathione, glutathione reductas, glutathione peroxidas in different types of tuberculosis
| Group | Serum protein carbonyl n mol/mg | Total blood glutathione mg/dl | Glutathione reductase u/g Hb | Glutathione peroxidase u/g Hb |
|---|
| Control(n=100) | 4.37 ± 0.15 | 74.55± 1.25 ** | 11.06 ± 1.21 ** | 62.57± 5.50 ** |
| Pulmonary tuberculosis |
| Category I untreated(n=100) | 6.65 ±0.22** | 60.21±3.5** | 5.69 ± 0.44 ** | 40.30± 3.27 ** |
| Category I after 6 month treatment(n=100) | 5.43 ±0.25** | 66.18± 3.14** | 7.07±0.50** | 47.17± 3.26 ** |
| Category II untreated (n=100) | 6.87 ±0.19** | 53.57± 3.24** | 5.17± 0.71 ** | 31.91± 6.20 ** |
| Category III untreated (n=100) | 7.08± 0.21** | 46.35± 2.82 ** | 3.77± 0.38 ** | 27.76± 4.56 ** |
| Extra pulmonary tuberculosis |
| Category I untreated(n=35) | 5.83 ±0.16** | 58.09± 3.47 ** | 6.69± 0.59 ** | 44.86± 2.90 ** |
| Category I after 6 month treatment (n=35) | 4.85 ±0.14** | 63.96± 3.80 ** | 7.43± 0.59 ** | 51.17± 4.57 ** |
P ≤ 0.001 – Highly significant,
P ≤ 0.05 – Significant; Values shown are Mean ±SD
Correlation between serum protein carbonyl and total blood glutathione, proteincarbonyl and glutathione reductas, protein carbonyl and glutathione peroxidas.
| Group | r-values Protein carbonyl/ Blood total glutathione | r-values Protein carbonyl/ Glutathione reductase | r-values Protein carbonyl/ Glutathione peroxidase |
|---|
| Pulmonary tuberculosis |
| Category I untreated(n=100) | -0.943** | -0.950** | -0.950** |
| Category I after 6 month treatment(n=100) | -0.921** | -0.970** | -0.963** |
| Category II untreated (n=100) | -0.966** | -0.923** | -0.953** |
| Category III untreated (n=100) | -0.936** | -0.890** | -0.878** |
| Extra pulmonary tuberculosis |
| Category I untreated (n=35) | -0.884** | -0.931** | -0.966** |
| Category I after 6 month treatment (n=35) | -0.965** | -0.924** | -0.971** |
P ≤ 0.05 – Significant
P ≤ 0.01 – Highly significant.
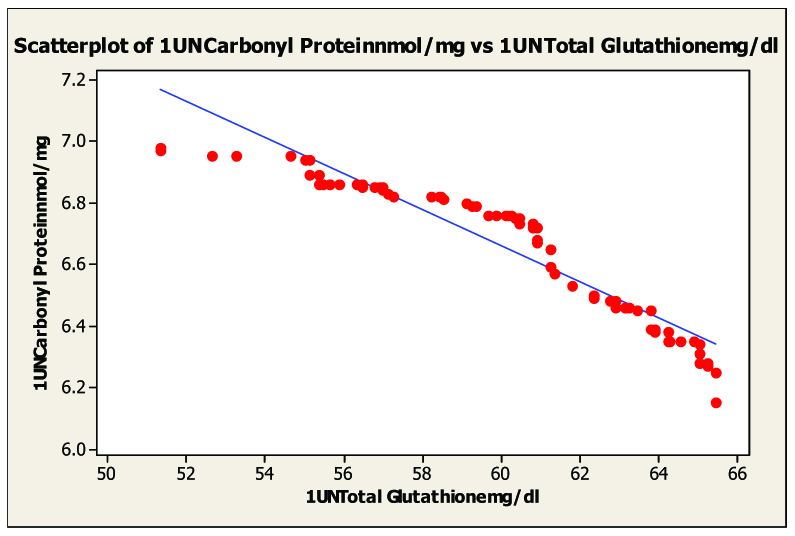
Scatter diagram of serum malondialdhyde and blood glutathione in untreated category I pulmonary tuberculosis untreated, r= –0.943
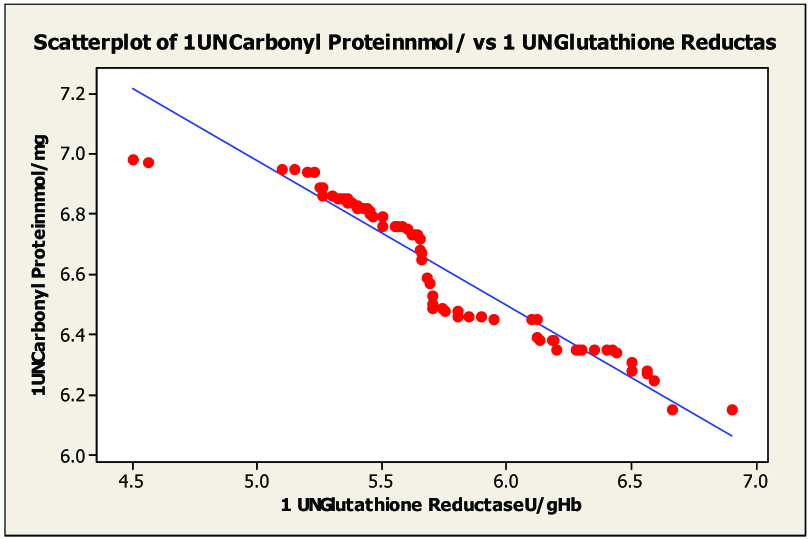
Scatter diagram of serum malondialdhyde and in glutathione reductas untreated category I pulmonary tuberculosis r= –0.950
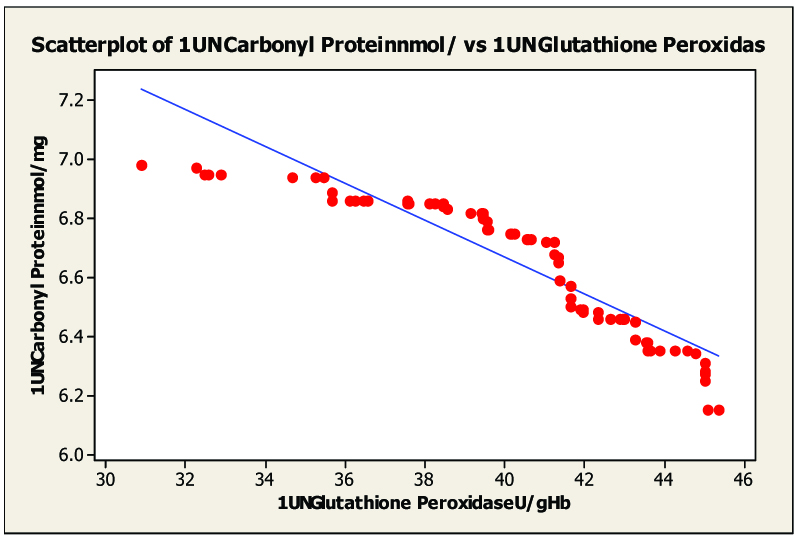
Scatter diagram of serum malondialdhyde and in glutathione peroxidas untreated category I pulmonary tuberculosis r= –0.950.
Discussion
All the control subjects, as well as the study groups, belonged to a poor nutritional status and a low socio economic status and they were from Mumbai, India. These subjects were also found to have common habits of sharing of clothes, food, drinking water containers, tea, etc. without broughening of spread of the infection. In the history taking, it was also found that they were travelling, sitting closer to one another (sharing of small space, which had led to the close contact breathing and the aerosol contamination), coughing and sneezing, which had led to the spread of the infection.
The present study was a comprehensive evaluation of the concentrations of the circulating antioxidants and the markers of oxidative stress in pulmonary tuberculosis and extra pulmonary tuberculosis patients in different categories with and without the anti-tuberculosis (DOTS) therapy as compared to the healthy human volunteers. According to our study, on carefully observing the mean values of the biochemical parameters, it was seen in category III, that there was a concomitant increase in the protein carbonyl content [13–15]. There were significant reductions in enzymatic glutathione peroxidase, glutathione reductase and non enzymatic antioxidants glutathione [16–18], which acted as primary internal antioxidants and played a buffering role, thus showing an increasingly protective effect. Several factors such as a low food intake, nutrient malabsorption and inadequate nutrient release from the liver, acute infections and an inadequate availability of the carrier molecules may influence the circulating antioxidant concentrations.
The serum protein carbonyl level was significantly increased (p< 0.001) and the levels of glutathione peroxidase, glutathione reductase and non enzymatic antioxidants glutathione were decreased in the category I patients with pulmonary and extra-pulmonary tuberculosis before the treatment. The levels of protein carbonyl and increase levels of glutathione peroxidase, glutathione reductase and total glutathione after six months treatment but not lowered and increased respectively as compared to normal control group [Table/Fig-1]. Protein oxidation, which leads to protein carbonyl formation, may change their physio-chemical properties like their charge, hydrophobicity, catalytic properties, etc. The introduction of carbonyl groups in proteins makes them susceptible to degradation by proteolytic enzymes, leading to deficiency of the proteins. The proteins may aggregate together and some proteins may become resistant to degradation, whereas some proteins cannot serve their function and hence, this modification can cause many pathological conditions. The modification of bacterial proteins and lipids; modification of bacterial DNA and the direct interaction with accessory protein targets result in enzymatic inactivation or other protein malfunctions; and the induction of macrophage apoptosis leads to intracellular mycobacterial killing [19].
On statistical evaluation [Table/Fig-2], the correlation between protein carbonyl and blood glutathione, glutathione peroxidase and glutathione reductase in pulmonary and extra-pulmonary tuberculosis showed a negative correlation in pulmonary tuberculosis [Table/Fig-3, 4 and 5] and extra-pulmonary tuberculosis. The correlation co-efficient was significantly different from zero and it was found to be statistically highly significant (two sided) in patients of pulmonary and extra-pulmonary tuberculosis. This clearly indicated that the elevated levels of protein carbonyl and decreases blood glutathione, glutathione peroxidase, glutathione reductase hence there is tight negative relationship between blood glutathione, glutathione peroxidase, glutathione reductase and protein carbonyl seen in tuberculosis patients. The oxidative modification of enzymes can have either mild or severe effects on the cellular or the systemic metabolism, depending on the percentage of the molecules that are modified and the chronicity of the modification.
The marked reduction in glutathione concentration and the greater susceptibility of erythrocytes for lipid peroxidation and haemolysis suggests the occurrence of oxidant stress in tuberculosis patients. Even treatment of antitubercular therapy after 6 month levels were not reached to that of the control group. The treatment of tuberculosis should be prolonged if equilibrium of oxidative stresses is not balanced [20].
Conclusion
The mean serum protein carbonyl levels were concomitantly increased and blood glutathione, glutathione peroxidase, glutathione reductase were decreased, in different categories of pulmonary tuberculosis and extra-pulmonary tuberculosis patients before treatment and levels were reached to normal subjects but not completely reached even after treatment of six months (Cat. I) those subjects need furthers few months more treatment even though they are sputum negative.
The changes in the levels are a reflection of the defense mechanisms and increased free radical activity in tuberculosis. Alarming signals of the tuberculosis infection warrant global attention for more extensive research and need rapid outcomes to control the massive rate of infection in the present situation. An in depth gene expression study at a molecular level is required to correlate the pathogenesis and the progression of multidrug resistance in tuberculosis disease with studies on protein carbonyl expression and glutathione expression status in individual subjects along with the drug treatment.
[1]. Flynn JL, Chan J, Immunology of tuberculosisAnnu. Rev. Immunol 2001 19:93-129. [Google Scholar]
[2]. Chevion M, Berenshtein E, Stadtman ER, sHuman studies related to protein oxidation: protein carbonyl content as a marker of damageFree Radic Res 2000 33:99-108. [Google Scholar]
[3]. Castegna A, Drake J, Methods in biological oxidative stress, carbonyl protein levels – An assessment of protein oxidationMethods in Pharmacology and Toxicology 2003 3:161-68. [Google Scholar]
[4]. Barbara S, Berlett Earl R, Stadtman Protein oxidation in aging, disease and oxidative stressThe Journal of Biological Chemistry 1997 272(15):20313-16. [Google Scholar]
[5]. Meister A, Methods in Systems BiologyMethods in Enzymology 1995 251:3-7. [Google Scholar]
[6]. Hafeman DG, Sunde RA, Hoekstra WG, Effect of dietary selenium on erythrocyte and liver glutathione peroxidase in the ratJ. Nutr. 1974 104:580-87. [Google Scholar]
[7]. Chu FF, The human glutathione peroxidase genes GPX2, GPX3, and GPX4 map to chromosomes 14, 5, and 19, respectivelyCytogenet Cell Genet 1994 66(2):96-98. [Google Scholar]
[8]. Carranza C, Juarez E, Torres M, Mycobacterium tuberculosis growth control by lung macrophages and CD8 cells from patient contactsAm J Resp Crit Care Medicine 2006 173:238-45. [Google Scholar]
[9]. Levine RL, Garland D, Determination of carbonyl content in oxidatively modified proteinsMethods in Enzymology 1990 186:464-78. [Google Scholar]
[10]. Beutler E, Improved method for the determination of blood glutathioneJ Lab Clin Med 1963 61:882-90. [Google Scholar]
[11]. Paglia DE, Valentine WN, Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidaseLab Clin Med 1967 :158-69. [Google Scholar]
[12]. Goldberg DM, Spooner RJ, Method for the determination of glutathione reductaseMethods of Enzymatic Analysis 1983 3(3):258-65. [Google Scholar]
[13]. Drancourt M, Raoult D, Palaeomicrobiology: current issues and perspectivesNature Review Microbiology 2005 3:23-35. [Google Scholar]
[14]. Khan MA, Tania M, Zhang D, Chen H, Antioxidant enzymes and cancerChin J Cancer Res 2010 22(2):87-92. [Google Scholar]
[15]. Hipkiss AR, Carnosine and protein carbonyl groups: A possible relationshipBiochemistry 2000 65(7):907-16. [Google Scholar]
[16]. Kulkarni A, Madrasi NA, Relationship of nitric oxide and protein carbonyl in tuberculosisIndian J Tuberc 2008 55(3):138-44. [Google Scholar]
[17]. Reddy YN, Murthy SV, Krishna DR, Prabhakar MC, Role of free radicals and antioxidants in tuberculosis patientsIndian J tuberc 2004 51:213-18. [Google Scholar]
[18]. Vijayamalini M, Manoharan S, Lipid peroxidation, Vitamins C, E and reduced glutathione levels in patients with pulmonary tuberculosisCell Biochem Funct 2004 22:19-22. [Google Scholar]
[19]. Andrade JA, Crow JP, Protein nitration, metabolites of reactive nitrogen species and inflammation in lung allograftsAm J Respir Crit Care Med 2000 161(6):2035-42. [Google Scholar]
[20]. Connolly LE, Edelstein PH, Ramakrishnan L, Why is long-term therapy required to cure tuberculosis?PLOS Medicine 2007 4:e120 [Google Scholar]