Hypokinetic-rigid syndrome, also known as Parkinson’s Disease (PD), is a progressive neurodegenerative disorder. Motor symptoms in patients can accelerate the decline in functional independence, particularly when associated with a sedentary lifestyle [1,2]. The worldwide prevalence of PD is reported to be nearly 6.3 million, with onset usually after 60 years of age. However, studies estimate that 5-10% of patients experience symptoms before the age of 40, with one in ten diagnosed before 50 years [2-5]. A deficiency of the neurochemical dopamine in the nigrostriatal system is the primary cause of PD [5].
Tremor is the most common cardinal motor symptom of PD, an involuntary, rhythmical, oscillatory movement of a body part [6]. Approximately 50% of patients experience tremors during the disease, while around 9% never do [7]. Classical pin-rolling, postural, or essential tremors are present in PD patients [8]. The classical pin-rolling tremor in PD presents with a frequency of 4-7 Hz and is commonly observed in the hands or arms [9,10]. Tremor appearance and frequency worsen with stress and anxiety, significantly impacting activity and participation [11]. The neurophysiological relationship in PD tremors is complex. While the relationship between stress, PD and tremors is not extensively studied, worsening tremors under psychological or physical stress are well-recognised in clinical practice.
It is hypothesised that the loss of dopaminergic cells inhibits motor thalamic nuclei, reducing cortical excitation and leading to tremors [12,13]. These pathological UE alterations exacerbate challenges in reaching, grasping and manipulating objects quickly and deftly for functional activities [13]. These upper limb deficits can limit employment, recreation and social activities, often causing difficulties with eating, drinking and self-care [14]. In addition to tremors, PD patients often experience motor blocks, or ‘freezing’ episodes - the sudden inability to initiate movement - during UE movements [15,16]. Freezing most commonly occurs during short, rapid movements involving index finger flexion and extension [17]. Recent research investigated the impact of movement complexity, amplitude and frequency during alternating flexion/extension movements of the index finger in individuals with PD, with and without auditory cueing [18]. In low-amplitude tasks, the patient group with freezing episodes showed the highest movement variability. This suggests that freezing may be related to UE movement variability and freezing during bimanual tasks. Amplitude and frequency adjustments may also impact UE function. These freezing episodes, combined with tremors, are disabling factors for UE function in people with PD. This study aimed to evaluate the impact of tremor on upper limb function in PD, correlating tremors (FTMS) with hand function (BPT and 9HPT).
Materials and Methods
A hospital-based observational cross-sectional correlational study was performed at Sri Krishna Neuro and General Hospital, Kasibugga, Andhra Pradesh, India for one year (September 2023-August 2024). Institutional Ethical Committee (IEC) approval (EC/2023-24/030) was obtained; the study forms part of a larger research project.
Inclusion criteria: Participants were diagnosed with primary PD [19,20], Hoehn and Yahr stages III-IV with tremor dominance [20], able to perform the postural tremor of the hand test and achieving a score of 2 or 3 (mild to moderate) [21,22], aged 40-60 years, medically stable and taking prescribed medication were included in the study.
Exclusion criteria: Participants who had secondary Parkinson’s syndrome, Parkinson’s Plus syndrome, severe co-morbidities (lung, heart, kidney, liver dysfunction, or cancer), severe psychological illness, or dementia were excluded from the study.
Sample size: Using G*Power 3.1.9.4 software, a sample size of 21 was calculated [19]. This sample size estimation for t-tests and point-biserial correlation used a one-tailed test, an effect size of 0.6, an α level of 0.05, and a power (1-β) of 0.95.
Study Procedure
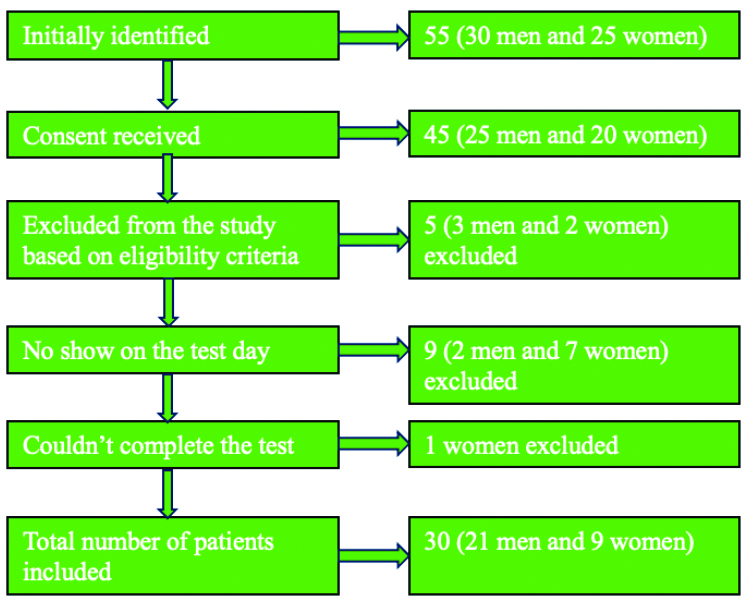
Fifty-five eligible subjects were recruited; 10 did not consent (various reasons, including time constraints). Of the 45 consenting patients, five were excluded (co-morbidities and non primary PD diagnosis). Nine participants did not attend the scheduled testing and no further contact was made. One woman could not complete the test due to severe tremor exacerbation. Thirty participants completed the study, exceeding the calculated minimum sample size. Patient screening is illustrated in [Table/Fig-1]. Study objectives and procedures were explained and consent was obtained.
Screening of patients for eligibility.
Assessments were conducted over 72 hours in increments to avoid fatigue. Measures were administered in a random order. The FTMS [23] scores tremor severity (0-4; 0=normal, 4=severely abnormal). The Bain and Findley Tremor (BFT) for Activities of Daily Living (ADL) [24] assesses ADL (0-3; higher scores indicate greater severity). The 9HPT measures time to completion (seconds) [25]. An assessor blinded to the study objective administered all three scales.
The FTMS is widely recommended for evaluating tremor in PD [23,24]. The 9HPT and BFT are reliable (ICC 0.92-0.95) and recommended for assessing hand function [24]. The FTMS comprises three subsections: Part A (resting tremor), Part B (tremor during action, e.g., handwriting) and Part C (functional difficulty). Scoring is done for each subsection, the total and a severity component. The scale has acceptable clinimetric properties [23,24]. The 9HPT is a reliable measure of hand function [25] and the BFT is a valid and reliable tool with acceptable clinimetric properties [24].
Statistical Analysis
All variables were entered into a spreadsheet. Central tendency and variability (mean, standard deviation, median, IQR) were calculated based on the measurement scale and data were tested for normality. Non parametric statistics (Spearman’s rank correlation coefficient) were used. Significance was set at p-value ≤0.05. All analyses were performed using Jamovi software.
Results
The included PD patients for the present study consisted of 30 patients, of whom 9 (30%) were females and 21 (70%) were males. The mean age was 56.5±3.83 years (males: 56.7±2.95 years; females: 55.9±5.6 years). The severity and chronicity of the disease are shown in [Table/Fig-2]. Descriptive statistics of the measurements are presented in [Table/Fig-3]. The Shapiro-Wilk test of normality showed a p-value <0.05 for all variables, indicating a non normal distribution. Therefore, Spearman’s rho was used to compute the correlation between scores of subsets (A, B, C) and total scores of the Fahn-Tolosa-Marín Tremor Rating Scale (FTMS; total and severity), the 9-Hole Peg Test (9HPT) and the Bain and Findley Tremor (BFT) for Activities of Daily Living (ADL). The results are shown in [Table/Fig-4]. Each FTMS subscore was correlated with scores on each hand in the 9HPT and the BFT scores. However, no correlation was found between tremor and hand function on either side, although a moderate correlation was observed with tremor-related ADL rating scores (BFT).
Severity and chronicity of the disease.
| Patients | Disease severity Hohn and Yahr scale (n) | Chronicity of disease (years since diagnosis) Mean±SD |
|---|
| III | IV |
|---|
| Men | 15 | 6 | 5.9±2.23 |
| Women | 5 | 4 | 7.11±1.76 |
| Total | 20 | 10 | 6.27±2.15 |
Descriptive statistics of the measurements.
| Measure | Measures of tremor | Measures of hand function |
|---|
| FTMS A | FTMS B | FTMS C | FTMS Total | FTMS severity | 9HPT- DS | 9HPT- NDS | BFT |
|---|
| Median | 62 | 26 | 20 | 109 | 75.7 | 25.2 | 27.2 | 71 |
| IQR | 4.75 | 6.25 | 2.75 | 4.75 | 3.30 | 6.79 | 6.87 | 2.75 |
FTMS: Fahn-Tolosa-Marín tremor rating scale; 9HPT-DS: 9 hole peg test dominant side; 9HPT-NDS: 9 hole peg test non-dominant side; BFT: Bain and findley tremor; ADL Scale; IQR: Inter-quartile range
Measures of association between scores of tremor rating instruments and hand function measurements.
| Tremor scale components | Spearman’s rho | p-value |
|---|
| 9 HPT-DS | 9 HPT-NDS | BFT |
|---|
| FTMS A | -0.03 | - | - | 0.86 |
| - | -0.2 | - | 0.89 |
| - | - | 0.31 | 0.09 |
| FTMS B | -0.29 | - | - | 0.12 |
| - | -0.26 | - | 0.17 |
| - | - | 0.03 | 0.89 |
| FTMS C | -0.06 | - | - | 0.76 |
| - | -0.02 | - | 0.93 |
| - | - | 0.31 | 0.09 |
| FTMS total | -0.22 | - | - | 0.23 |
| - | -0.16 | - | 0.39 |
| - | - | 0.46 | 0.01* |
| FTMS severity | -0.22 | - | - | 0.24 |
| - | -0.15 | - | 0.24 |
| - | - | 0.46 | 0.01* |
FTMS: Fahn-Tolosa-Marín tremor rating scale; 9HPT: DS: 9 hole peg test dominant side; 9HPT: DS: 9 hole peg test non-dominant side; BFT: Bain and findley tremor; ADL Scale, *: Statistically significant (p≤0.05)
Discussion
This study investigated the impact of tremors on UE function in PD patients. Thirty patients were recruited, with tremors evaluated using the FTMS, hand function assessed with the 9HPT and ADL assessed using the BFT. No significant correlations were found between FTMS and 9HPT scores; however, a discernible moderate correlation was observed between FTMS and BFT scores. Although comparable numbers of males and females were initially approached, more male patients participated. This was not considered a limitation, as the objective was to evaluate the association between tremors and hand function using standardised tests without gender bias.
In contrast, a previous study [26] found that women are more likely than men to exhibit tremors as the initial symptom of PD, with a sex ratio of approximately 2:1 at diagnosis. During the preclinical stage, women may experience fewer symptoms due to the protective effect of oestrogens [26]. Haaxma CA et al., [26] demonstrated a link between the effect of oestrogens and varying iron levels in women and men [26]. Solla P et al., used the Unified Parkinson’s Disease Rating Scale (UPDRS) to assess sex differences in motor symptoms and found that women are more likely to have poorer tremors and motor instability as early PD symptoms, as well as a lower UPDRS instability score [27].
Participants were aged 60 years or less. Therefore, the results apply only to this age group. The additional effects of ageing on hand function were not investigated, although previous literature suggests a relationship between ageing and tremor-affected hand function in older persons with PD [28,29]. Tremors are more pronounced with later age of onset and advancing age may increase the rate of disease progression and its manifestations, including tremors [29].
Multiple studies have found that hand function decreases with PD, affecting dexterity, ADL performance and grip strength [30-32]. Paz T da SR et al., reported that hand function is related to the severity of movement-related symptoms, including tremor [33]. The current study’s relatively younger participants (disease onset before 60 years) with moderate severity may explain the lack of correlation between tremors and standardised hand function assessment using the 9HPT. Similarly, Delier HB et al., reported no correlation between tremor severity and 9HPT scores [34].
However, this study found a discernible moderate correlation between FTMS and BFT scores (tremor-related ADL). Delier HB et al., found a significant positive correlation between tremor severity and disabilities of the arm, shoulder and hand in individuals with essential tremor, concluding that hand skills and UE functionality were affected [34]. This supports the correlation between tremor severity and ADL. The lack of correlation between 9HPT and tremors may be due to the test’s inability to discern changes in this population. Solaro C et al., reported floor and ceiling effects in the 9HPT in mildly and severely affected stroke patients [35], consistent with the present study findings. The BFT has been reported to be sensitive to changes in tremor characteristics [24], which may explain its association with FTMS severity and total score.
Performance bias was minimised by randomising the order of test administration. The sample size exceeded the minimum computed sample size, increasing the study’s power to 0.99. The use of established tests with good validity is another strength. Limitations include the cross-sectional design, which prevented conclusions about how patients typically perform regular hand activities due to tremors. Although efforts were made to administer tests when medication effects were minimal, patients frequently reported earlier or later than scheduled, potentially impacting results and contributing to the lack of significance between FTMS and 9HPT scores.
Limitation(s)
The study suggests that tremor extent is related to ADL performance in individuals with PD but not to standardised hand function tests, implying that ADL should be assessed clinically rather than using standardised non functional hand function tests. Limitations include the relatively younger PD population and cross-sectional study design; future research should investigate the effect of tremors on hand function longitudinally and using patient-reported outcome measures.
Conclusion(s)
This study determined the impact of tremor on UE function in PD. No significant correlations were observed between tremors (FTMS) and 9HPT; however, a discernible moderate correlation was observed between tremors (FTMS) and tremor-related ADL performance (BFT). Future research could explore the potential correlation between PD motor and non motor symptoms, reduced quality of life and cognitive impairment.
FTMS: Fahn-Tolosa-Marín tremor rating scale; 9HPT-DS: 9 hole peg test dominant side; 9HPT-NDS: 9 hole peg test non-dominant side; BFT: Bain and findley tremor; ADL Scale; IQR: Inter-quartile range
FTMS: Fahn-Tolosa-Marín tremor rating scale; 9HPT: DS: 9 hole peg test dominant side; 9HPT: DS: 9 hole peg test non-dominant side; BFT: Bain and findley tremor; ADL Scale, *: Statistically significant (p≤0.05)