Cryptogenic Organising Pneumonia (COP) is a rare Interstitial Lung Disease (ILD) characterised by a distinctive histological pattern without a known cause. It was earlier referred to as bronchiolitis obliterans organising pneumonia and is brought on by alveolar damage, which causes organised granulation tissue to form, resulting in the blockage of alveoli and bronchioles. If left untreated, the obstructions may cause progressive respiratory failure. This case report describes a 65-year-old female who exhibited respiratory symptoms like low-grade fever, dry cough, poor appetite and loss of weight for about one month, alongside radiological characteristics resembling lung malignancy. The imaging studies showed a dense, irregular opacity with spiculations in the left lung field, leading to a potential misdiagnosis. Comprehensive evaluation, including fibreoptic bronchoscopy which aided in obtaining a Transbronchial Lung Biopsy (TBLB) sample for histopathological examination, revealed alveolar spaces containing foamy macrophages, neutrophils and intra-alveolar plugs of fibroblastic tissue all typical of COP, leading to its diagnosis. Despite the initial challenges posed by the puzzling radiological features, a prompt and accurate diagnosis rooted in histopathology allowed for timely corticosteroid treatment. This case highlights the diagnostic challenges posed by the overlap of radiological attributes between COP and lung malignancy, emphasising the need for careful assessment to avoid multiple unnecessary invasive procedures and delays in treatment. It also underlines the utility of a nuanced histopathological study. Awareness of COP’s clinical and radiological similarities to malignancy and a comprehensive diagnostic approach in such cases is essential for reliable diagnosis, effective patient management and good prognosis.
Biopsy, Bronchiolitis obliterans lung diseases, Corticosteroids, Fibrosis
Case Report
A 65-year-old female presented with complaints of low-grade fever, dry cough, loss of appetite and weight (approximately 5 kilograms) for about one month in a secondary healthcare setting. The patient was a non smoker but had a significant biomass fuel exposure history (firewood cooking). Her past medical history was unremarkable with no known co-morbidities. She had no history of recent travel, and there was no family history of respiratory diseases or malignancies.
On examination, the patient appeared weak but was not in acute distress. She was vitally stable, with a blood pressure of 130/76 mmHg, a pulse rate of 87 beats per minute, a respiratory rate of 21 breaths per minute, and a peripheral saturation of oxygen of 97% without any external oxygen support. Specific respiratory system examination revealed coarse crepitations throughout inspiration and expiration over the left infra-axillary area on auscultation of lung fields. Multiple differential diagnoses like pneumonia, pulmonary Tuberculosis (TB), pneumoconiosis, lung cancer and ILD were considered.
A complete blood count revealed an increased total leukocyte count (14,900 cells/cumm) with neutrophilic predominance. Liver and renal function tests were within normal limits. Work-up for any possible immunosuppression like infection by human immunodeficiency viruses or hepatitis B virus antigens turned out to be non reactive. There were no haematuria or albuminuria in routine investigations on urine. Blood and sputum cultures showed no growth of any organisms. Ziehl–Neelsen staining of sputum was also negative for acid-fast bacilli.
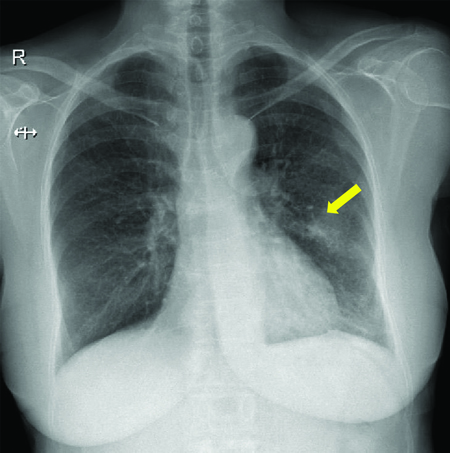
Her symptoms were persistent and progressed rapidly. A plain chest radiograph was taken in posteroanterior view which showed a left-sided mid-zone inhomogeneous opacity [Table/Fig-1].
A plain chest radiograph taken in posteroanterior view showing a left-sided mid-zone inhomogeneous opacity (yellow arrow).
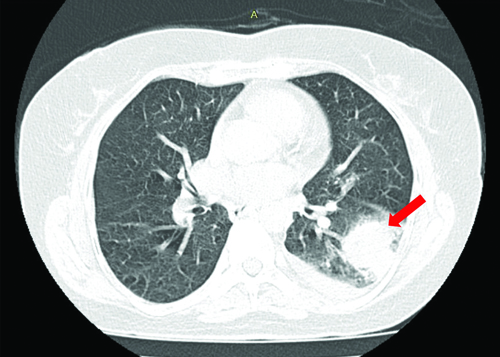
For a better understanding of this lesion, it was followed-up with a High-Resolution Computed Tomography (HRCT) of the thorax which revealed an irregular dense solid space occupying lesion with large soft tissue component and suspicious mild spiculations in the left lower lobe of the lung. This lesion was also surrounded by multiple tiny satellite nodules, thus favouring neoplastic aetiology [Table/Fig-2].
HRCT of thorax showing an irregular dense solid space occupying lesion with features favouring neoplastic aetiology in the lower lobe of the left lung (red arrow).
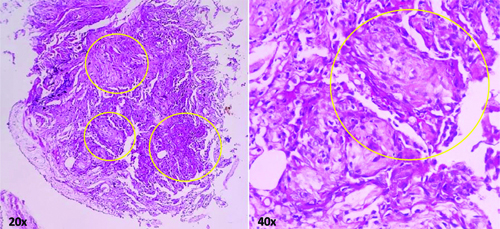
At this point, the patient was referred to a tertiary healthcare setting for further evaluation and management. A fibreoptic bronchoscopy to obtain TBLB and bronchoalveolar lavage was planned and executed. It revealed a normal tracheobronchial tree. Bronchoalveolar lavage specimen was negative for growth of any bacteria (Mycobacterium tuberculosis) or any atypical cells. Histopathological examination of the TBLB sample using haematoxylin and eosin stains showed alveolar spaces with plenty of foamy macrophages, few neutrophils and intra-alveolar plugs of fibroblastic tissue. Mild interstitial fibrosis was also observed. These features were suggestive of COP. Granulomas, fungal elements or neoplastic cells were not seen [Table/Fig-3].
Histopathological examination of the TBLB sample using haematoxylin and eosin stains on 20x and 40x magnifications revealing fibroblastic plugs in the alveolar spaces (yellow circles).
On revisiting the radiological presentation and correlating it with the histopathological studies, it was diagnosed as a case of COP and the patient was started on oral prednisolone 30 milligrams per day for four weeks and then tapered to 15 milligrams per day for the next four weeks.
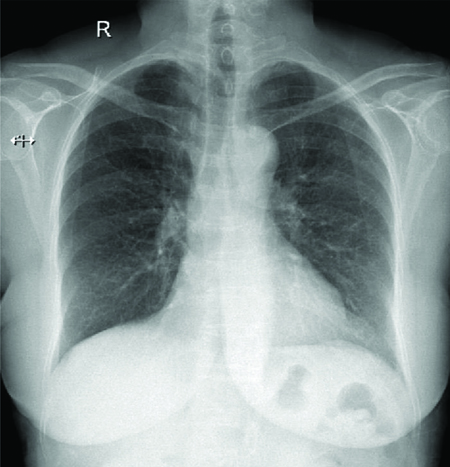
On follow-up after two months, serial chest radiographs showed resolution of the inhomogeneous opacities [Table/Fig-4].
A plain chest radiograph on follow-up showing near complete resolution of inhomogeneous opacities.
The patient showed significant clinical recovery. This case highlights the importance of considering COP in patients with radiological findings suggestive of malignancy, particularly when clinical and histopathological findings are not congruent.
Discussion
The COP is a relatively rare form of ILD that can be challenging to diagnose due to its diverse clinical and radiological features [1]. This case illustrates how COP can closely mimic lung cancer, highlighting the importance of careful diagnostic evaluation [2].
The overlap in clinical symptoms and radiological findings between COP and lung malignancy is well recognised. In a similar case reported by Jain S et al., this overlap was clearly noted in a patient of Indian descent with COP presenting with cough, shortness of breath, and fever, which are also quite common in lung malignancies [3]. Radiological imaging in COP may reveal nodular opacities, mass-like consolidations, or ground-glass opacities, which are characteristics that often lead to a suspicion of malignancy [4].
Damage to the alveolar basement membrane and type II pneumocytes, caused by various intrinsic and extrinsic factors, is the key to the development of organising pneumonia [5]. While vascular endothelial cells remain intact, alveolar integrity disruption results in the leakage of fibrotic inflammatory exudate into the alveolar lumen and adjacent structures. Inflammatory cells and fibroblasts migrate, transforming into myofibroblasts that bind to components of the extracellular matrix [4].
The lung’s architecture remains preserved, and epithelial cells proliferate for re-epithelialisation [4]. Key cytokines, including Interleukin-6 (IL-6) and transforming growth factor-β, are elevated in serum and bronchoalveolar lavage fluid obtained from organising pneumonia patients, highlighting the role of inflammatory cells in disease progression [6]. During resolution, inflammatory cells decrease, and myofibroblast clusters form collagen foci. Disruptions in this process can lead to the scarring variant of OP. In COP, the inflammatory process occurs simultaneously, often preserving lung architecture, while allowing for fibrotic lesions [4].
Radiologically, numerous alterations in the COP are visible on HRCT [7]. Typically, COP is well-defined from the surrounding parenchyma with a lobular pattern close to the bronchovascular structures. This is a crucial imaging hint that helps distinguish it from other infectious processes, such as bronchopneumonia, which has a blurry or fluctuating boundary around healthy lung parenchyma [7]. The most common imaging finding (more than 80%) is parenchymal consolidation, which is frequently accompanied by an air bronchogram [2]. Most of the time, parenchymal consolidations alter in size and location as the disease progresses [8].
In this particular case, the presence of a mass-like consolidation on the HRCT scan, coupled with the patient’s history of exposure to biomass fuel (firewood cooking), initially suggested a diagnosis of lung malignancy. Case reports in literature like Yao HM et al., describing an abnormal straight, sharp and enhanced lesion in chest CT planned for lung cancer screening, turned out to be COP on histopathological examination of a pulmonary biopsy sample [9]. Thus, the decision to opt for histopathological examination, rooted in an understanding of existing research, in this case, was momentous and impactful.
Differentiating COP from lung malignancy is crucial because the treatment approaches for these conditions are significantly different. While lung cancer often requires interventions like surgery, chemotherapy, or radiotherapy depending on its stage, COP is primarily managed with corticosteroids [10]. Misdiagnosis can result in unnecessary invasive procedures, such as biopsies or surgeries, and delays in initiating appropriate treatment, as was the situation in this case where the primary focus was on excluding malignancy.
Conclusion(s)
This case of COP highlights the diagnostic challenges presented by its similarity to lung malignancy. The initial misdiagnosis can lead to unnecessary invasive procedures and delays in appropriate treatment, emphasising the need for comprehensive evaluation, including histopathological examination. The identification of characteristic histological features, such as granulation tissue and foamy macrophages, confirmed COP and enabled the initiation of corticosteroid therapy. This case underscores the importance of considering COP in differential diagnoses for lung masses, particularly when risk factors or malignancy indicators are absent. Awareness of the overlapping clinical and radiological features of COP and lung malignancy is crucial for an effective patient management and avoiding burdensome interventions. Accurate diagnosis is essential not only for appropriate treatment but also for alleviating the physical and emotional stress associated with potential malignancy. This case serves as a reminder of the complexities in diagnosing ILDs and the necessity for vigilance in distinguishing benign from malignant processes.
[1]. Chandra D, Maini R, Hershberger DM, Cryptogenic Organizing PneumoniaIn: StatPearls [Internet] 2022 Treasure Island (FL)StatPearls Publishing [Google Scholar]
[2]. Tiralongo F, Palermo M, Distefano G, Vancheri A, Sambataro G, Torrisi SE, Cryptogenic organizing pneumonia: Evolution of morphological patterns assessed by HRCTDiagnostics (Basel) 2020 10(5):26210.3390/diagnostics1005026232365469PMC7277545 [Google Scholar] [CrossRef] [PubMed]
[3]. Jain S, Rathore YS, Jindal A, Joshi V, Cryptogenic organizing pneumonia mimicking a mass lesion: An unusual caseIndian J Respir Care 2019 8(2):124-26.10.4103/ijrc.ijrc_40_18 [Google Scholar] [CrossRef]
[4]. Radzikowska E, Fijolek J, Update on cryptogenic organizing pneumoniaFront Med 2023 10:114678210.3389/fmed.2023.114678237153105PMC10157489 [Google Scholar] [CrossRef] [PubMed]
[5]. Reddy BSK, Ghewade B, Jadhav U, Wagh P, Scleroderma associated with organising pneumonia and polyarthritis: A report of a rare caseCureus 2024 16(1):e5288610.7759/cureus.52886 [Google Scholar] [CrossRef]
[6]. Radzikowska E, Roży A, Jaguś P, Wiatr E, Gawryluk D, Chorostowska-Wynimko J, Cryptogenic organizing pneumonia: IL-1β, IL-6, IL-8, and TGF-β1 serum concentrations and response to clarithromycin treatmentAdv Exp Med Biol 2016 911:77-85.10.1007/5584_2016_22326987326 [Google Scholar] [CrossRef] [PubMed]
[7]. Kloth C, Thaiss WM, Beer M, Bösmüller H, Baumgartner K, Fritz J, The many faces of cryptogenic organizing pneumonia (COP)J Clin Imaging Sci 2022 12(1):2910.25259/JCIS_208_202135769096PMC9235424 [Google Scholar] [CrossRef] [PubMed]
[8]. Raghu G, Meyer KC, Cryptogenic organising pneumonia: Current understanding of an enigmatic lung diseaseEur Respir Rev 2021 30(161):21009410.1183/16000617.0094-202134407978PMC9488952 [Google Scholar] [CrossRef] [PubMed]
[9]. Yao HM, Zuo W, Wang XL, Zhang W, Findings on cryptogenic organizing pneumonia: A case report and literature reviewJ Int Med Res 2020 48(4):30006052092006810.1177/030006052092006832321352PMC7180307 [Google Scholar] [CrossRef] [PubMed]
[10]. Taniguchi Y, Arai T, Tsuji T, Sugimoto C, Tachibana K, Akira M, Cryptogenic organizing pneumonia: Clinical outcomes of 60 consecutive casesJ Thorac Dis 2024 16(5):312910.21037/jtd-24-22538883617PMC11170430 [Google Scholar] [CrossRef] [PubMed]