A Silent Dissection Complicating Myocardial Infarction: A Case Report
Adithya Shelley1, Akshat Sharma2, Jostol Pinto3
1 Department of Cardiology, Father Muller Medical College, Mangaluru, Karnataka, India.
2 Department of Cardiology, Father Muller Medical College, Mangaluru, Karnataka, India.
3 Associate Professor, Department of Cardiology, Father Muller Medical College, Mangaluru, Karnataka, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Adithya Shelley, Department of Cardiology, Father Muller Medical College, Father Muller’s Road, Kankanady, Mangaluru-575002, Karnataka, India.
E-mail: adithyabsp@gmail.com
A Left Ventricular (LV) pseudoaneurysm develops when free myocardial wall rupture is contained by an adherent layer of overlying pericardium and scar tissue. It is a rare mechanical complication seen in 0.2% of patients post Myocardial Infarction (MI). Furthermore, it remains a challenge to diagnose and differentiate it from a true aneurysm due to overlapping clinical presentations and echocardiographic findings. A 40-year-old male patient presented with ST Elevation Myocardial Infarction (STEMI) and received guideline directed management. However, he was later found to have a pansystolic murmur at the apex and an early diastolic murmur over the left lower sternal border (to and fro murmur) on auscultation. An echocardiogram had subsequently revealed a dissecting pseudoaneurysm of the left ventricle and Contrast-enhanced Computed Tomography (CECT) confirmed the presence of an anterior dissecting pseudoaneurysm. This is an atypical presentation of a niche post MI complication due to its asymptomatic presentation, atypical location and unique anatomy despite prompt revascularisation. Furthermore, the authors emphasise the importance of cardiac auscultation on a day-to-day basis and high index of suspicion to ensure early diagnosis and management of such a silent, calamitous complication.
Echocardiogram, Left ventricular pseudoaneurysm, True aneurysm
Case Report
A 40-year-old hypertensive male with no other co-morbidities presented with acute, severe retrosternal chest pain and diaphoresis lasting over five hours. He had no dyspnoea or fatigue. There was no family history of any cardiac illness. On examination, he had a regular pulse at 102 beats/minute, a blood pressure of 76/46 mmHg and a respiratory rate of 20/min. Auscultation revealed no cardiac murmurs at presentation. The electrocardiogram showed ST elevations in anterior leads and elevated cardiac troponin [Table/Fig-1]. The echocardiogram showed hypokinesia of the basal and mid anterior and anterolateral segments and distal interventricular septum. A diagnosis of anterior wall STEMI was made. He was administered aspirin 325 mg, clopidogrel 300 mg, atorvastatin 80 mg and intravenous heparin 5000 U. A coronary angiogram was done which revealed 80% stenosis in the proximal and mid Left Anterior Descending artery (LAD) with maintained flow to the distal LAD (slow flow). Primary Percutaneous Coronary Intervention (PCI) with stenting was done to LAD. The patient felt better post-procedure and had no residual chest pain or new-onset dyspnoea. However, on the next morning, auscultation revealed a grade 4 pansystolic murmur at the apex (with a systolic thrill) and a grade 3 early diastolic murmur over the left lower sternal border (to and fro murmur). The echocardiogram was repeated and it now revealed a dissecting pseudoaneurysm of the left ventricle [Table/Fig-2,3]. However, the LV systolic function was well preserved. CECT was done to define its extent and it showed a contained rupture of the LV septum and free wall in the superior-anteromedial aspect of the LV at the basal interventricular septum and along the LV outflow tract below the level of the ascending aortic wall. The dimensions of the pseudoaneurysm were 65×61×35 mm in its body and 7.5×7.8 mm at its neck [Table/Fig-3,4] with irregular margins and containing thrombus. It was contained within the pericardial cavity with a thin partial rim of myocardium. However, there was a co-existing circumferential haemopericardium 11 mm thick [Table/Fig-5]. Nevertheless, he remained haemodynamically stable. The patient was not keen on suggested surgical intervention due to its entailed risks and likely poor outcomes and was discharged on request. He then followed-up two weeks later, still well preserved with normal jugular venous pressure and no dyspnoea. However, the patient did not seek further medical attention and passed away three weeks later due to sudden cardiac death at home.
Electrocardiogram showing anterior wall STEMI.
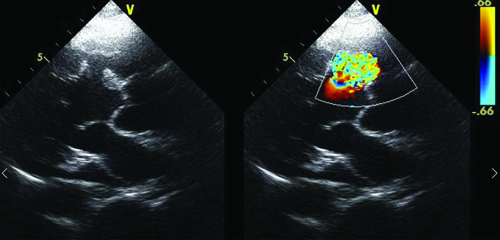
Echocardiography in parasternal long axis view showing the entry tear of the dissecting LV pseudoaneurysm (left panel) in the basal interventricular septum with turbulent flow (right panel) detected by colour doppler.
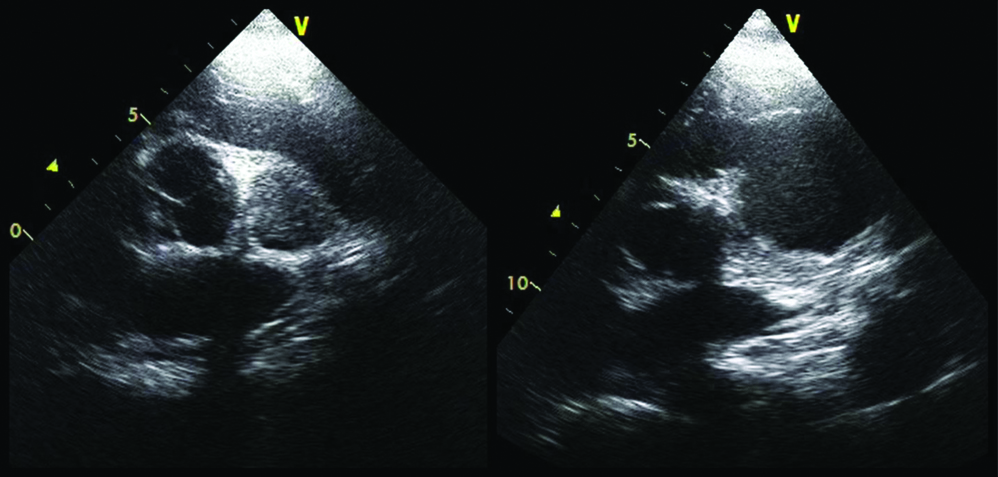
Echocardiography in parasternal short axis view showing thrombus in the LV false lumen (left panel) adjacent to the aortic valve and a large false luminal cavity (right panel).
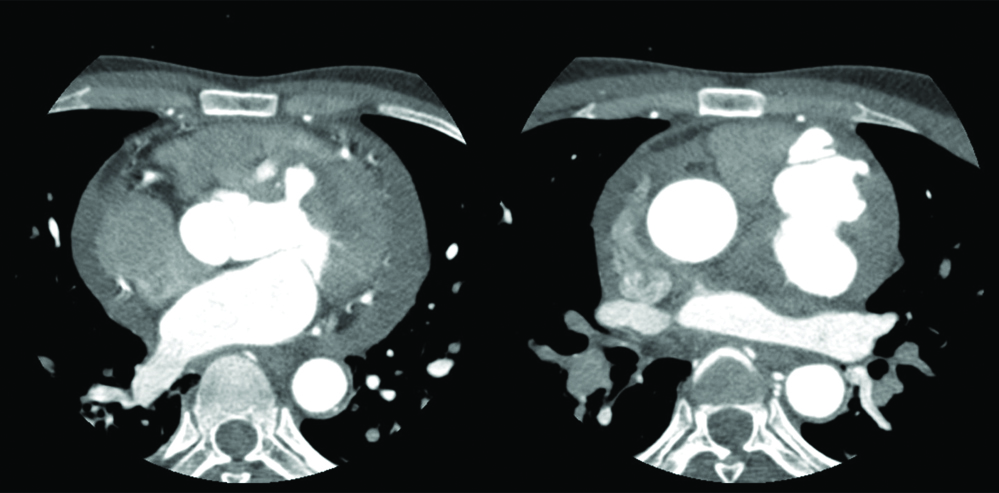
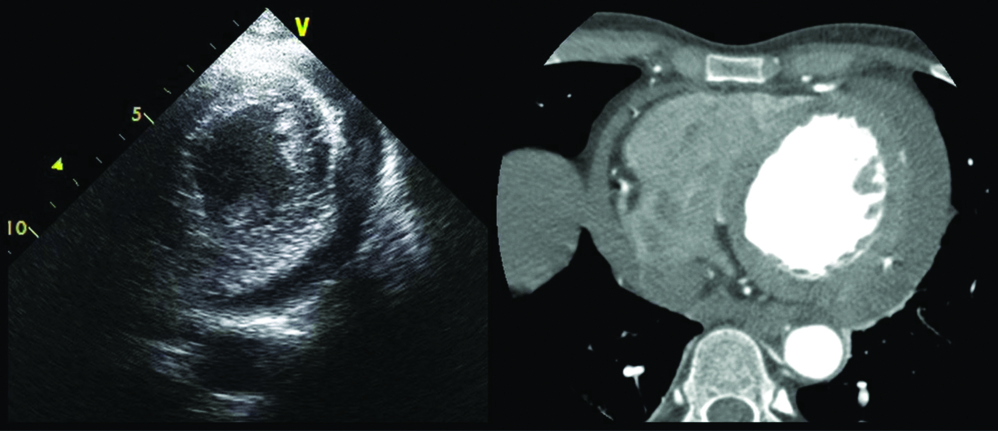
Contrast-enhanced Computed Tomography (CECT) outlining the extent of the LV pseudoaneurysm as 7.5×7.8 mm at its neck (left panel) and 65×61×35 mm in its body (right panel) constituting neck:body ratio of diameters of 0.12.
Circumferential haemopericardium seen on echocardiography (with thrombus) (left panel) and on computed tomography (right panel).
Discussion
The incidence of mechanical complications following acute MI has reduced significantly from 6% in the prethrombolysis era to 0.2%-1% in the reperfusion era [1]. However, once a mechanical complication does occur in this clinical setting, a corresponding decline in the in-hospital mortality rate has not been observed and has continued to remain at approximately 38.7-40.5% [1]. LV pseudoaneurysms represent a rare, potentially fatal complication seen in 0.2%-0.3% of patients following acute MI based on a retrospective study of 1,050 patients [2]. A pseudoaneurysm is bounded by pericardium, pericardial adhesions, thrombus, and/or scar tissue whereas true LV aneurysms are additionally bounded by myocardium. It is often difficult to distinguish the two due to similar clinical presentations and echocardiographic findings. LV pseudoaneurysms are more common following inferolateral/posterior MIs as opposed to true ventricular aneurysms, which frequently occur following anterior MI [3].
Infero-postero-lateral pseudoaneurysms are more common (84.6% of all cardiac pseudoaneurysms); anterior pseudoaneurysms are rarer due to incomplete adhesion by pericardium and a higher propensity for complete free wall rupture and death before diagnosis is made [3]. This highlights the rarity of the present case as the patient had developed an anterior wall pseudoaneurysm and yet was asymptomatic and stable. Furthermore, since anterior wall aneurysms are more commonly true rather than pseudoaneurysms [4], this atypically located aneurysm could have easily been misinterpreted as a true aneurysm. Transmural MI is the leading cause of LV pseudoaneurysms (accounting for 55%) followed by cardiac surgery, and trauma [3]. The risk factors for developing LV pseudoaneurysms include older age, hypertension, inferior and lateral MI, first MI, late presentation of MI, and delayed myocardial revascularisation [3]. LV pseudoaneurysms often present with chest pain, progressive dyspnoea, syncope, arrhythmias, embolism and sudden cardiac arrest; but can be asymptomatic in 10% as seen in the patient. The present case report underscores that an LV pseudoaneurysm can occur even in the present era of thrombolysis and PCI and further stealthily manifest not necessarily at the time of presentation. On examination, soft heart sounds, pericardial friction rub and a classical to and fro murmur is found in about 50%-70% of patients [3]. The hallmark anatomical features of LV pseudoaneurysms are a narrow neck at the site of the rupture with an abrupt transition in dimensions with the ratio of the neck diameter to maximum cavity diameter being less than 0.5. The patient had an LV pseudoaneurysm with a more abrupt transition with a neck diameter to cavity diameter ratio of 7.8:65, nearly 0.12 [Table/Fig-3].
Management of pseudoaneurysms involves repair with patch closure and thrombectomy when feasible and if the benefits of surgery surpass the risk of rupture. A recent single institutional cohort study over five years showed that surgical repair was superior to medical management of postinfarction pseudoaneurysm due to no in-hospital mortality, and good functional status during follow-up over 18.5 months [5]. Another study reported a surgical mortality rate of 23% over three decades [3]. The Cleveland Clinic reported a larger group of patients with a 20% mortality rate [6]. The medically treated group had poor survival of 36% over a follow-up of two years which correlated with high mortality (48%) seen in medically treated patients from other studies [3,7]. A similar case presented with dyspnoea and chest tightness 20 days after anterior wall MI, auscultation revealed the classical to and fro murmur and echocardiogram had confirmed the presence of a large apical pseudoaneurysm. However, the patient passed away two weeks later due to poor prognosis and conservative management [8]. Despite conservative management being a potential treatment option for asymptomatic, small (<3 cm), and stable LV pseudoaneurysms [7,9] there still exists a significant risk of fatal rupture, sudden cardiac death in all medically treated patients, despite having survived the acute phase of acute MI and being asymptomatic. LV pseudoaneurysms are rarely accompanied by other mechanical complications Shimono H et al., reported a rare case of LV pseudoaneurysm with ventricular septal rupture, which was successfully managed by surgical repair. The distal LAD lesion in this patient is implicated in pseudoaneurysm formation and the mid LAD lesion for septal perforation [10].
Conclusion(s)
The patient was diagnosed with a rare structural complication of MI; however, it was further atypical as it had occurred despite revascularisation. Another rare feature was its anatomical location: anterior position of an LV pseudoaneurysm. Further, the patient remained asymptomatic until discharge and first outpatient follow-up. This does highlight the importance of cardiac auscultation in diagnosis of structural complications of MI not just at presentation but even following revascularisation on a day-today basis until the patient is discharged from the hospital.
Author Declaration:
Financial or Other Competing Interests: None
Was informed consent obtained from the subjects involved in the study? Yes
For any images presented appropriate consent has been obtained from the subjects. Yes
Plagiarism Checking Methods: [Jain H et al.]
Plagiarism X-checker: Sep 20, 2024
Manual Googling: Dec 10, 2024
iThenticate Software: Dec 12, 2024 (6%)
[1]. Elbadawi A, Elgendy IY, Mahmoud K, Barakat AF, Mentias A, Mohamed AH, Temporal trends and outcomes of mechanical complications in patients with acute myocardial infarctionJ Am Coll Cardiol Intv 2019 12:1825-36.10.1016/j.jcin.2019.04.03931537282 [Google Scholar] [CrossRef] [PubMed]
[2]. Montrief T, Davis WT, Koyfman A, Long B, Mechanical, inflammatory, and embolic complications of myocardial infarction: An emergency medicine reviewAm J Emerg Med 2019 37(6):1175-83.10.1016/j.ajem.2019.04.00330987913 [Google Scholar] [CrossRef] [PubMed]
[3]. Frances C, Romero A, Grady D, LV pseudoaneurysmJ Am Coll Cardiol 1998 32:557-61.10.1016/S0735-1097(98)00290-39741493 [Google Scholar] [CrossRef] [PubMed]
[4]. Sharma A, Kumar S, Overview of left ventricular outpouchings on cardiac magnetic resonance imagingCardiovasc Diagn Ther 2015 5:464-70. [Google Scholar]
[5]. Zhong Z, Song W, Zheng S, Liu S, Surgical and conservative treatment of post-infarction left ventricular pseudoaneurysmFront Cardiovasc Med 2022 9:80151110.3389/fcvm.2022.80151135155628PMC8829002 [Google Scholar] [CrossRef] [PubMed]
[6]. Atik FA, Navia JL, Vega PR, Gonzalez-Stawinski GV, Alster JM, Gillinov AM, Surgical treatment of postinfarction left ventricular pseudoaneurysmAnn Thorac Surg 2007 83:526-31.10.1016/j.athoracsur.2006.06.08017257982 [Google Scholar] [CrossRef] [PubMed]
[7]. Yeo TC, Malouf JF, Oh JK, Seward JB, Clinical profile and outcome in 52 patients with cardiac pseudoaneurysmAnn Intern Med 1998 128:299-305.10.7326/0003-4819-128-4-199802150-000109471934 [Google Scholar] [CrossRef] [PubMed]
[8]. Bai W, Tang H, Left ventricular pseudoaneurysm following acute myocardial infarctionAnatol J Cardiol 2018 20(6):E10-E11.10.14744/AnatolJCardiol.2018.39001PMC6287443 [Google Scholar] [CrossRef]
[9]. Prâtre R, Linka A, Jenni R, Turina MI, Surgical treatment of acquired left ventricular pseudoaneurysmsAnn Thorac Surg 2000 70:553-57.10.1016/S0003-4975(00)01412-010969679 [Google Scholar] [CrossRef] [PubMed]
[10]. Shimono H, Kajiya T, Inoue H, Ueno M, Takaoka J, Atsuchi Y, Left ventricular pseudoaneurysm with ventricular septal rupture due to anterior ST-segment elevation myocardial infarctionIntern Med 2019 58(13):1901-05.10.2169/internalmedicine.2147-1831257277PMC6663542 [Google Scholar] [CrossRef] [PubMed]