Autoimmune Hepatitis (AIH) is a chronic inflammatory liver disease characterised by the presence of circulating autoantibodies, hypergammaglobulinemia and interface hepatitis on histological examination. AIH in Human Immunodeficiency Virus (HIV)-positive patients presents a unique diagnostic and therapeutic challenge due to the complex interplay between immune activation and immunodeficiency. Treatment considerations in such cases require a careful balance between immunosuppression for AIH and maintaining adequate immune function for HIV control. A 67-year-old female with a 30-year history of well-controlled HIV infection presented with jaundice and haematemesis. Initial evaluation revealed hypotension and abnormal liver function tests. Imaging studies demonstrated portal hypertension with oesophageal varices, requiring endoscopic variceal ligation. Serological work-up showed positive antinuclear antibodies (1:320), elevated Immunoglobulin G (IgG) levels (2270 mg/dL) and positive Anti-mitochondrial-M2 (AMA-M2), with a normal Cluster Differentiation (CD4) count (802/mm3). A liver biopsy confirmed AIH with advanced fibrosis (score 6/6) and significant portal plasmacytosis. The patient was initiated on steroid therapy, which led to clinical improvement. At the three-month follow-up, liver function tests showed significant improvement, with total bilirubin decreasing from 6.56-3.56 mg/dL, AST from 166-80 U/L and ALT from 152-79 U/L. The patient maintained stable HIV control throughout the treatment period. The present case demonstrates that AIH can be successfully managed with standard immunosuppressive therapy in HIV-positive patients with well-controlled disease, highlighting the importance of considering autoimmune conditions in the differential diagnosis of liver dysfunction in HIV patients.
Fibrosis, Human immunodeficiency virus, Immunosuppression, Jaundice, Liver cirrhosis, Plasmacytosis
Case Report
A 67-year-old female housewife presented to the Emergency Department with the acute onset of jaundice, manifesting as yellowish discoloration of the sclera and skin for 15 days, accompanied by haematemesis for two days. The patient had a 30-year history of HIV infection, which had been well managed with antiretroviral therapy. Notably, she had no history of alcohol consumption and her family history was negative for liver disease.
On initial examination, the patient was found to be hypotensive due to haematemesis but showed no signs of chronic liver disease, such as flapping tremors, ascites, or palpable masses. Upon admission, immediate stabilisation measures were implemented, including intravenous fluid resuscitation. The patient was started on intravenous pantoprazole and empiric antibiotic therapy. This initial management approach aimed to address the acute presentation of upper Gastrointestinal (GI) bleeding and prevent potential complications.
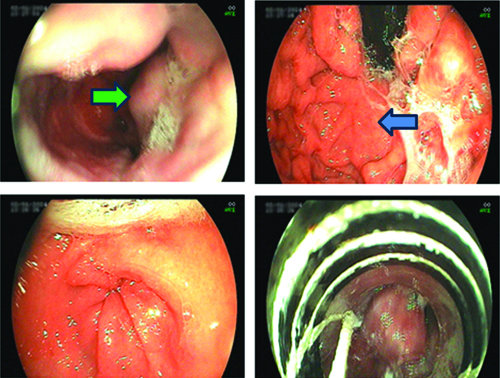
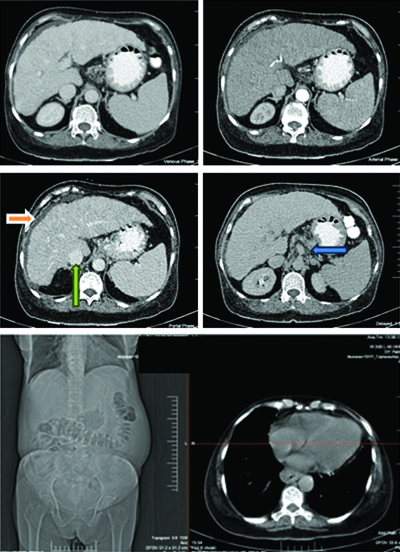
Comprehensive imaging studies were performed to evaluate the extent of liver involvement. Abdominal sonography revealed altered hepatic parenchymal echotexture with surface nodularity, a contracted gallbladder and minimal ascites. Upper gastrointestinal endoscopy demonstrated oesophageal varices and portal hypertensive gastropathy with dilated fundic channels, necessitating endoscopic variceal ligation [Table/Fig-1]. Contrast-enhanced Computed Tomography (CECT) confirmed the presence of liver parenchymal disease, along with portal vein dilation, portosystemic collaterals, minimal ascites and splenomegaly [Table/Fig-2].
Upper GI endoscopy image showing oesophageal varices (green arrow) and dilated fundic channels in a mossaic pattern (blue arrow).
Contrast-enhanced CT (axial view) showing ascites (orange arrow) and subtle nodularity of liver (green arrow) and portal vein dilatation (blue arrow).
Laboratory investigations revealed significant abnormalities in liver function tests, with elevated total bilirubin (6.56 mg/dL), predominantly direct bilirubin (4.30 mg/dL). Transaminases were moderately elevated, with Aspartate Aminotransferase (AST) at 166 U/L and Alanine Aminotransferase (ALT) at 152 U/L. The patient also demonstrated elevated alkaline phosphatase (320 U/L) and Gamma-glutamyl Transferase (GGT) levels (278 U/L). Hematological parameters showed pancytopenia [Table/Fig-3].
Laboratory investigations.
| Lab parameters | Result | Biological reference interval |
|---|
| Haemoglobin | 8.3 g/dL | 11.6-15.00 g/dL |
| Total leucocyte count | 3500 cells/μL | 4000-10000 cells/μL |
| Platelet count | 86000 cells/μL | 150000-400000 cells/μL |
| Haematocrit | 36% | 35-44.90% |
| MCV | 90 fL | 78.2-97.9 fL |
| RDW | 14% | 11.2-16.1% |
| Total bilirubin | 6.56 mg/dL | 0.22-1.20 mg/dL |
| Direct bilirubin | 4.30 mg/dL | upto 0.5 mg/dL |
| Indirect bilirubin | 1.41 mg/dL | 1-1.0 mg/dL |
| AST | 166 U/L | 8-43 U/L |
| ALT | 152 U/L | 7-45 U/L |
| ALP | 320 U/L | 35-104 U/L |
| GGT | 278 U/L | 8-61 U/L |
MCV: Mean corpuscular volume; RDW: Red cell distribution width; AST: Aspartate aminotransferase; ALT: Alanine aminotransferase; ALP: Alkaline phosphatase; GGT: Gamma-glutamyl transferase
Immunological and serological work-up yielded significant findings. Hepatotropic viral markers were negative, while autoimmune markers showed positive Antinuclear Antibody (ANA) (1:320), positive AMA-M2 and weakly positive Anti-smooth Muscle Antibody (ASMA). Serum IgG was elevated at 2270 mg/dL, while Liver Kidney Microsomal type 1 (LKM-1) and anti-Soluble Liver Antigen (SLA) antibodies were negative. The patient’s HIV status was well-controlled, as evidenced by a CD4 count of 802/mm3.
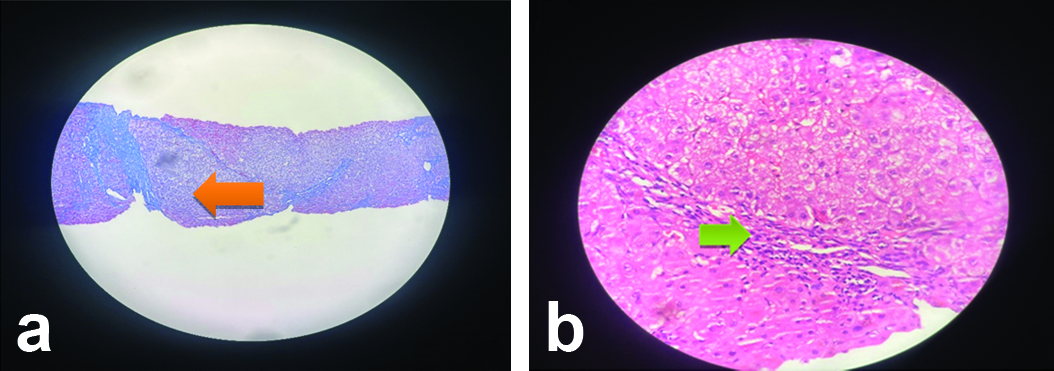
A liver biopsy was performed to establish a definitive diagnosis. The biopsy revealed portal fibrosis with chronic inflammation, predominantly lymphocytic infiltration and significant portal plasmacytosis with focal perivenulitis [Table/Fig-4]. These findings were consistent with AIH. The necroinflammatory score was 5/18 and the fibrosis score was 6, indicating established cirrhosis.
Histopathology showing portal fibrosis with chronic inflammation; a) Bridging fibrosis (orange arrow) (Masson trichome stain, 400x); b) Lymphoplasmocytic infiltration (green arrow) (H&E, 400x).
Based on the clinical presentation, laboratory findings and histological features, a diagnosis of AIH was established. The patient was initiated on steroid therapy. After a three-day observation period with clinical improvement, the patient was discharged on Tablet (Tab) prednisolone 40 mg once daily, Tab propranolol hydrochloride 20 mg once daily, Tab pantoprazole 20 mg once daily and a multivitamin tablet once daily, along with appropriate follow-up instructions.
At the three-month follow-up, the patient showed significant clinical and biochemical improvement. Follow-up laboratory studies demonstrated improvement in liver function parameters, with total bilirubin decreasing to 3.56 mg/dL, AST to 80 U/L, ALT to 79 U/L and alkaline phosphatase to 118 U/L. Hematological parameters showed slight improvement in haemoglobin (8.8 g/dL), while maintaining stable leucocyte and platelet counts.
The present case highlights the successful diagnosis and management of AIH in a patient with long-standing HIV infection, demonstrating that appropriate immunosuppressive therapy can be safely and effectively administered in such cases when carefully monitored.
Discussion
The AIH is a chronic inflammatory liver disease characterised by the presence of circulating autoantibodies, hypergammaglobulinemia and interface hepatitis on histological examination [1]. The coexistence of AIH and HIV infection presents a unique diagnostic and therapeutic challenge, particularly given the opposing nature of these conditions, one characterised by immune hyperactivity and the other by immunodeficiency [1]. The present case provides valuable insights into the successful management of AIH in a patient with long-standing HIV infection, contributing to the limited literature on this topic.
The presentation of a patient with jaundice and haematemesis is consistent with previous reports [2], though the present case patient demonstrated a more severe initial presentation with portal hypertension and oesophageal varices. Ofori E et al., reported a similar case of a 45-year-old HIV-positive patient who presented with jaundice, albeit without complications of portal hypertension [2]. The advanced presentation in the current case might be attributed to the delayed recognition of AIH symptoms, possibly due to the focus on HIV management.
The immunological profile in the present patient showed high titres of ANA (1:320) and elevated IgG levels (2270 mg/dL), similar to findings reported by Parekh S et al., in their case series [3]. However, this patient also demonstrated positive AMA-M2 antibodies, suggesting a potential overlap syndrome. This finding aligns with observations made by Cottura N et al., who noted that autoimmune liver diseases in HIV patients might present with atypical serological patterns [4].
The diagnostic complexity in the present case mirrors challenges described by Chaiteerakij R et al., who noted that elevated IgG and positive autoantibodies are common in HIV patients due to polyclonal B-cell stimulation [5]. However, the present case patient’s clear histological findings and response to therapy confirmed the diagnosis.
Recent findings by Laurenda O et al., document three cases of AIH in HIV patients, all presenting after immune reconstitution with advanced hepatic fibrosis, demonstrating successful treatment with immunosuppression and improvement in liver fibrosis, which reinforces the authors observation about the importance of restored immune function in AIH development [6].
The successful response to steroid therapy in the present case, evidenced by significant improvement in liver function tests at three months, supports findings from Virot E et al., who demonstrated the safety and efficacy of immunosuppression in HIV patients with autoimmune conditions [7]. The reduction in transaminases (AST from 166-80 U/L and ALT from 152-79 U/L) is comparable to response rates seen in non HIV AIH patients, as reported in the European Association for the Study of the Liver (EASL) Clinical Practice Guidelines (CPGs) [8].
The management approach in the present case addressed both the acute presentation (haematemesis) and the underlying AIH, demonstrating that standard AIH treatment protocols can be effectively applied to HIV-positive patients. This aligns with recommendations from Durazzo M et al., who advocate for similar treatment approaches in both HIV and non-HIV AIH patients, provided HIV is well-controlled [9].
Conclusion(s)
The present case report highlights the successful diagnosis and management of AIH in a long-term HIV survivor with well-controlled disease. Despite the complex interplay between immune dysregulation in HIV and autoimmune hyperactivity in AIH, the present case demonstrates that standard immunosuppressive therapy can be safely and effectively administered to HIV-positive patients when the viral infection is well managed. Early recognition, accurate diagnosis and a multidisciplinary approach are essential for achieving positive outcomes in such challenging cases. The present case report contributes valuable insights into the growing body of literature on HIV and AIH, emphasising the importance of considering autoimmune liver diseases in the differential diagnosis of liver dysfunction in individuals infected with HIV.
MCV: Mean corpuscular volume; RDW: Red cell distribution width; AST: Aspartate aminotransferase; ALT: Alanine aminotransferase; ALP: Alkaline phosphatase; GGT: Gamma-glutamyl transferase
[1]. Mack CL, Adams D, Assis DN, Diagnosis and management of autoimmune hepatitis in adults and children: 2019 Practice guidance and guidelines from the American Association for the study of liver diseasesHepatology 2020 72(2):671-722.10.1002/hep.3106531863477 [Google Scholar] [CrossRef] [PubMed]
[2]. Ofori E, Ramai D, Ona MA, Reddy M, Autoimmune hepatitis in the setting of human immunodeficiency virus infection: A case seriesWorld J Hepatol 2017 9(36):1367-71.10.4254/wjh.v9.i36.136729359021PMC5756727 [Google Scholar] [CrossRef] [PubMed]
[3]. Parekh S, Spiritos Z, Reynolds P, Parekh T, HIV and autoimmune hepatitis: A case series and literature reviewJ Clin Transl Hepatol 2017 6(2)10.4172/2254-609X.100057 [Google Scholar] [CrossRef]
[4]. Cottura N, Kinvig H, Grañana-Castillo S, Wood A, Siccardi M, Drug-drug interactions in people living with HIV at risk of hepatic and renal impairment: Current status and future perspectivesJ Clin Pharmacol 2022 62(7):835-46.Epub 2022 Feb 810.1002/jcph.202534990024PMC9304147 [Google Scholar] [CrossRef] [PubMed]
[5]. Chaiteerakij R, Sanpawat A, Avihingsanon A, Treeprasertsuk S, Autoimmune hepatitis in human immunodeficiency virus-infected patients: A case series and review of the literatureWorld J Gastroenterol 2019 25(35):5388-402.Available from: https://dx.doi.org/10.3748/wjg.v25.i35.538810.3748/wjg.v25.i35.538831558881PMC6761245 [Google Scholar] [CrossRef] [PubMed]
[6]. Laurenda O, Anneka P, Giovanni V, Adele M, Max W, Yvonne G, Autoimmune Hepatitis in people living with HIV: A case series and review of literatureOpen AIDS J 2023 17:e18746136230731010.2174/18746136-v17-230825-2023-15 [Google Scholar] [CrossRef]
[7]. Virot E, Duclos A, Adelaide L, Miailhes P, Hot A, Ferry T, Autoimmune diseases and HIV infection: A cross-sectional studyMedicine (Baltimore) 2017 96(4):e576910.1097/MD.000000000000576928121924PMC5287948 [Google Scholar] [CrossRef] [PubMed]
[8]. European Association for the Study of the LiverEASL Clinical Practice Guidelines: Autoimmune hepatitis [published correction appears in J Hepatol. 2015;63(6):1543-44J Hepatol 2015 63(4):971-1004.10.1016/j.jhep.2015.06.03026341719 [Google Scholar] [CrossRef]
[9]. Durazzo M, Lupi G, Scandella M, Ferro A, Gruden G, Autoimmune hepatitis treatment in the elderly: A systematic reviewWorld J Gastroenterol 2019 25(22):2809-18.10.3748/wjg.v25.i22.280931236003PMC6580347 [Google Scholar] [CrossRef] [PubMed]