Acquired Immunodeficiency Syndrome (AIDS) is caused by a retrovirus called HIV, which affects the immune system, leading to a spectrum of opportunistic infections, neurological disorders and unusual malignancies. It is estimated that nationally, there were approximately 2.349 million (1.798 million - 3.098 million) people living with HIV (PLHIV) in 2019, with an adult (15-49 years) HIV prevalence of 0.22% (0.17 - 0.29%) [1].
The HIV affects almost all major organ systems, including the Central Nervous System (CNS). The virus can infiltrate the CNS in the early stages of infection [2]. While it does not directly target neurons, HIV is often linked to structural and functional abnormalities in the brain [3-6], which can result in neurocognitive impairment. Neurocognitive dysfunction may hinder social and work-related interactions, decrease adherence to antiretroviral therapy, elevate the risk of additional health conditions and ultimately impair quality of life [7].
Cognition encompasses the mental processes used in understanding, evaluating, learning, perceiving, identifying, recalling, thinking and interpreting, which collectively shape our awareness of the environment. It is fundamental to gaining knowledge, forming beliefs and attitudes and engaging in decision-making and problem-solving [8]. Among various cognitive areas, HIV infection primarily impacts motor skills, attention, processing speed, executive function and memory [9].
Multiple studies suggest that the prevalence of cognitive disorders among people with HIV remains between 20% and 50%, even with effective antiretroviral therapy [10,11]. This indicates that nearly half of the approximately 35 million PLHIV worldwide, as estimated by UNAIDS [12], may face the risk of developing cognitive issues, including HAD.
The most recent diagnostic criteria, established by Antinori A et al., in 2007 and widely referred to as the Frascati criteria, categorise HAND into three distinct types: ANI, MND and HAD. These classifications are determined by three factors: performance on neuropsychological assessments, the presence or absence of functional decline and the exclusion of other potential causes for the symptoms [13]. The CNS HIV Antiretroviral Therapy Effects Research (CHARTER) study found that individuals diagnosed with ANI at baseline were two to six times more likely to develop symptomatic HAND during the three-year follow-up compared to those without baseline neurocognitive impairments [10].
The Mini-mental State Examination (MMSE), developed by Folstein MF et al., in 1975, is a widely used brief tool for evaluating cognitive function in elderly individuals and those with neurological impairments [14]. Neurocognitive function can be assessed using various methods, including the MMSE and the 3MS [15-17]. The 3MS is an expanded version of the MMSE designed to improve its ability to detect mild cognitive impairment and to offer a more comprehensive evaluation of cognitive abilities across various domains. Previous research has shown that the 3MS more effectively predicts functional outcomes [17].
The 3MS incorporates four additional tasks—date and place of birth, word fluency, similarities and delayed word recall—along with enhanced scoring guidelines and an expanded scoring range, with a maximum score of 100 points compared to 0 to 30 in the MMSE. This extension was designed to improve the tool’s sensitivity, reliability, validity and capacity to assess a broader spectrum of cognitive skills and difficulty levels. Although the 3MS takes longer to administer, it demonstrates superior psychometric qualities, including improved sensitivity and specificity compared to the MMSE [18-20].
The HAND is thus a significant cause of morbidity in PLHIV. However, it is important to highlight that a recent meta-analysis on structural brain changes in individuals with HIV has revealed grey and white matter atrophy, along with subcortical atrophy, even in those who are well treated [21]. There are limited studies assessing cognitive function in HAART-naïve patients using the 3MS and its relationship with CD4 count [22,23]. Therefore, the present study aimed to evaluate cognitive function in HAART-naïve patients using the 3MS and to associate it with the immunological status of these patients.
Materials and Methods
The present cross-sectional study was conducted among 120 PLHIV who were not on HAART and attended the outpatient and inpatient General Medicine Department of hospitals affiliated with Bangalore Medical College and Research Institute, Bengaluru, Karnataka, India, from November 2019 to May 2021. After obtaining approval and clearance from the Institutional Ethics Committee (IEC: ACA/DCD/SYN/BMC-B/PG/2019-20), patients who fulfilled the inclusion criteria were enrolled in the study after providing informed consent.
Inclusion criteria: Patients aged 18 to 60 years with newly diagnosed HIV positivity confirmed by the Enzyme-linked Immunosorbent Assay (ELISA) method and who were not on HAART were included in the study.
Exclusion criteria: Patients with signs of meningeal irritation, elevated intracranial pressure, altered sensorium, major opportunistic infections of the brain in the past three years, a history of head injury, stroke, previous depression, or those who could not comprehend the 3MS were excluded from the study.
Sample size calculation: The sample size was calculated based on the formula provided by Kumar S et al., N=(Zα/2)2*p*(1-p)/E2, where p is 0.21, 1-α is the Confidence interval, Z is the value associated with confidence (1.28), E is the absolute precision (a value less than p) equal to 0.05. The calculated sample size was N=109, which was approximated to 120 [22].
Study Procedure
The 3MS is an enhanced version of the MMSE, designed to improve the sensitivity of the MMSE [15]. The 3MS assesses cognitive function through various components, each contributing to the maximum score of 100 points. These components include date and place of birth (5 points), registration (3 points), mental reversal (7 points), first recall (9 points), temporal orientation (15 points) and spatial orientation (5 points), which evaluate memory and orientation. Language and verbal skills are assessed through naming (5 points), similarities (6 points), repetition (5 points) and the ability to read and obey (3 points). Additional cognitive abilities are measured by identifying a four-legged animal (10 points), writing (5 points), copying two pentagons (10 points), following three-staged commands (3 points) and second recall (9 points). Together, these components provide a comprehensive evaluation of cognitive function, making the 3MS a valuable tool for assessing cognitive impairment. The total score for the 3MS ranges from 0 to 100. Previous studies have demonstrated that a cut-off score of 79 on the 3MS yields sensitivity and specificity for detecting cognitive impairment of 98-100% and 70-81%, respectively. Based on this evidence, a cut-off score of 79 was selected to define cognitive impairment, with individuals scoring below 79 considered cognitively impaired [19,22,24].
Demographic data, clinical history, examination findings and investigations were collected. Patients were assessed for cognitive function using the 3MS and were staged according to the Frascati criteria [13]. The clinical diagnosis, opportunistic infections and CD4 count at presentation were noted. The standard of care was not affected, delayed, denied, or modified for the purpose of the present study.
Statistical Analysis
The Statistical Package for Social Sciences (SPSS) version 20.0 (IBM SPSS Statistics, IBM Corp., Armonk, NY, USA, released 2011) was used to perform the statistical analysis. Data were entered into an Excel spreadsheet. Descriptive statistics for the explanatory and outcome variables were calculated using the mean and standard deviation for quantitative variables and frequency and proportions for qualitative variables. Inferential statistics, such as the Chi-square test, were used for categorical variables. The level of significance was set at 5%.
Results
A total of 120 patients with HIV who were not on HAART were included in the study. Baseline clinical characteristics are presented in [Table/Fig-1]. The maximum number of patients in the study were in the age group of 36-45 years 34 (28.3%) patients, followed by the age group of 26-35 years 31 (25.8%) patients. In the present study, 77 (64.2%) patients were males, 42 (35.0%) patients were females and 1 (0.8%) patient identified as transgender. Additionally, 23 (19.2%) patients had diabetes mellitus as a co-morbidity, followed by hypertension in 15 (12.5%) patients and healed pulmonary tuberculosis in 6 (5%) patients.
Baseline and clinical characteristics of patients.
| Characteristics | n (%) |
|---|
| Age (in years) |
| 18-25 years | 9 (7.5) |
| 26-35 years | 31 (25.8) |
| 36-45 years | 34 (28.3) |
| 46-55 years | 28 (23.3) |
| >55 years | 18 (15) |
| Gender |
| Female | 42 (35) |
| Male | 77 (64.2) |
| Transgender | 1 (0.8) |
| Risk of HIV |
| Heterosexual | 113 (94.2) |
| MSM* | 7 (5.8) |
| Baseline CD4 Count (cells/mm3) |
| <100 | 31 (25.8) |
| 101 to 200 | 28 (23.3) |
| 201 to 300 | 18 (15) |
| 300 to 400 | 12 (10) |
| 400 to 500 | 10 (8.3) |
| >500 | 21 (17.5) |
| Co-morbidities |
| Diabetes mellitus | 23 (19.2) |
| Hypertension | 15 (12.5) |
| Hypothyroidism | 3 (2.5) |
| Hyperthyroidism | 2 (1.7) |
| Chronic kidney disease | 3 (2.5) |
| Healed pulmonary tuberculosis | 6 (5) |
| Chronic obstructive pulmonary disease | 1 (0.8) |
| Pancreatitis | 2 (1.7) |
| Carcinoma cervix | 1 (0.8) |
*Men who have sex with men
The patients were categorised according to the WHO Staging of HIV [Table/Fig-2]. There was a statistically significant association (p-value=0.001) between CD4 count and the Frascati criteria in the present study [Table/Fig-3]. The prevalence of HAND was found to be approximately 50.8% (n=61) among the patients with HIV. The prevalence rates of ANI, MND and HAD in the current study were 29.16%, 35% and 35.83%, respectively. It was noted that 50 (41.6%) patients with a CD4 count of <250 had a 3MS score of <79, while 18 patients (15%) had a CD4 count >500. Among patients with a CD4 count of 250-500, 23 patients (19%) had a 3MS score of >79 and 7 (5.8%) patients had a 3MS score of <79. In patients with a CD4 count >500, 18 (15%) patients had a 3MS score of >79 and 4 (3.3%) patients had a 3MS score of <79 [Table/Fig-4]. There was a statistically significant difference (p<0.01) in the distribution of CD4 counts among the 3MS scoring groups.
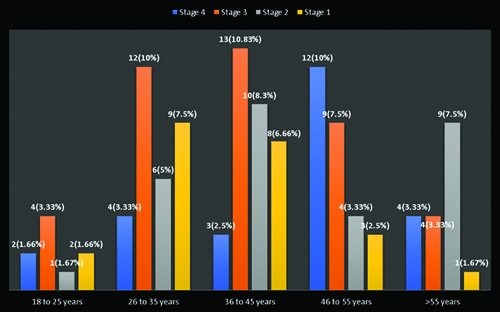
Distribution of age with WHO HIV clinical stage.
Distribution of CD4 count with Frascati criteria.
| CD4 count | Frascati criteria | Total |
|---|
| ANI* | HAD+ | MND# |
|---|
| <100 | 6 (17.1%) | 21 (50.0%) | 4 (9.3%) | 31 (25.8%) |
| 101 to 200 | 8 (22.9%) | 7 (16.7%) | 13 (30.2%) | 28 (23.3%) |
| 201 to 300 | 5 (14.3%) | 5 (11.9%) | 8 (18.6%) | 18 (15.0%) |
| 301 to 400 | 4 (11.4%) | 1 (2.4%) | 7 (16.3%) | 12 (10.0%) |
| 401 to 500 | 1 (2.9%) | 4 (9.5%) | 5 (11.6%) | 10 (8.3%) |
| 501 to 1000 | 11 (31.4%) | 4 (9.5%) | 6 (14.0%) | 21 (17.5%) |
Chi-square value-29.21; p value-0.001; *ANI: Asymptomatic neurocognitive impairment; +HAD: HIV-associated dementia; #MND: Mild neurocognitive disorder
Distribution of CD4 count with 3MS score.
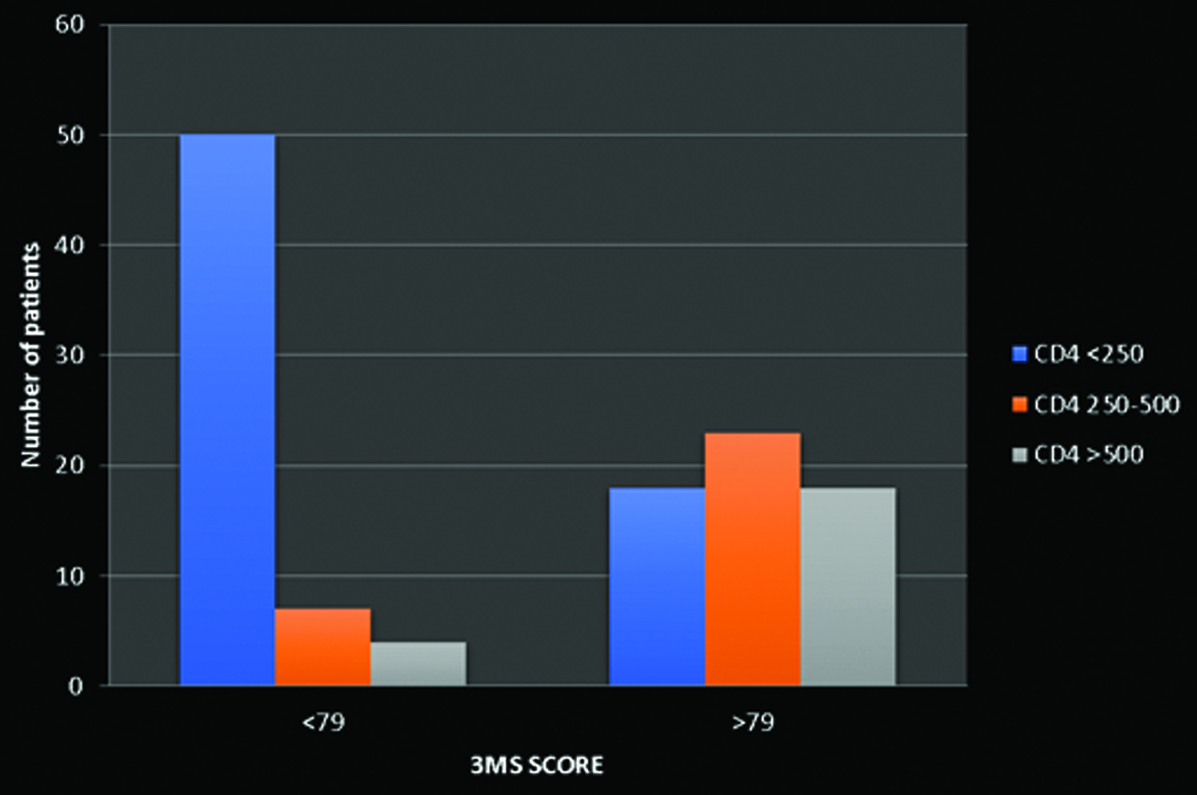 |
| <79 | >79 |
| CD4 <250 | 50 (41.6%) | 18 (15%) |
| CD4 250-500 | 7 (5.8%) | 23 (19%) |
| CD4 >500 | 4 (3.3%) | 18 (15%) |
The opportunistic infections present in patients is shown in [Table/Fig-5]. The most common opportunistic infection was oral candidiasis and pulmonary tuberculosis, affecting 29 (24.2%) patients, followed by disseminated tuberculosis in 18 (15%) patients. The mean distribution of the components of the 3MS is shown in [Table/Fig-6]. Out of the total 15 parameters of the 3MS, all the parameters were significantly below the maximum values for each parameter (p-value=0.01). The maximum decrease from normal value was observed in the similarities category (45.85%, mean score 3.79±1.73), followed by mental reversal (43%, mean score 3.99±1.56), read and obey (42.33%, mean score 1.73±1.24), first recall (41.10%, mean score 5.30±2.03) and second recall (40.33%, mean score 5.37±1.85).
Distribution of the subjects based on opportunistic infections.
| Variables | n (%) |
|---|
| Oral candidiasis | 29 (24.2) |
| Pulmonary TB | 29 (24.2) |
| Disseminated TB | 18 (15) |
| Syphilis | 12 (10) |
| HIV Retinopathy | 12 (10) |
| PCP | 10 (8.3) |
| UTI | 10 (8.3) |
| Herpes | 6 (5) |
| Hepatitis B | 6 (5) |
| Chronic suppurative otitis media | 5 (4.2) |
| Tinea corporis | 4 (3.3) |
| Oesophageal candidiasis | 4 (3.3) |
| Bacterial sepsis | 3 (2.5) |
| Post COVID-19 | 3 (2.5) |
| Genital candidiasis | 2 (1.7) |
| Genital warts | 2 (1.7) |
| HIV peripheral neuropathy | 2 (1.7) |
| Histoplasmosis | 2 (1.7) |
| Gullain-Barre syndrome | 1 (0.8) |
| HIV nephropathy | 1 (0.8) |
COVID-19: Coronavirus disease-2019
Mean distribution of components of 3MS. All components of 3MS contributing to a maximum score of 100 points.
| Components of 3MS | Mean±SD | Percentage of decrease |
|---|
| Date and place of birth (5) | 3.44±1.249 | 31.2% |
| Registration (3) | 2.74±0.587 | 8.6% |
| Mental reversal (7) | 3.99±1.558 | 43% |
| First recall (9) | 5.30±2.028 | 41.1% |
| Temporal orientation (15) | 13.17±1.440 | 12.2% |
| Spatial orientation (5) | 3.93±1.059 | 21.4% |
| Naming (5) | 3.87±0.448 | 14.1% |
| Four-legged animal (10) | 7.28±1.415 | 27.2% |
| Similarities (6) | 3.79±1.734 | 45.85% |
| Repetition (5) | 4.93±0.282 | 1.4% |
| Read and obey (3) | 1.73±1.235 | 42.33% |
| Writing (5) | 3.89±0.786 | 22.2% |
| Copying two pentagons (10) | 7.82±1.316 | 21.8% |
| Three staged commands (3) | 2.83±0.508 | 5.66% |
| Second recall (9) | 5.37±1.847 | 40.33% |
Discussion
The HIV-related neurological involvement is frequently linked to cognitive impairment and formal neurocognitive tests conducted on most HIV patients across the world have yielded poor outcomes [25]. Among the various methods used for neurocognitive testing, the 3MS has been found to be superior according to previous studies [16,17]. In the study group of 120 patients, the majority were males (64%), while 35% were females. This finding is consistent with previous studies [26,27]. In a study conducted by Reddy SG et al., 57.7% of participants were male and 42.3% were female [26]. This indicates that the disease is more prevalent in the male population, possibly due to a higher likelihood of acquiring the disease through high-risk behaviors. Additionally, it may be that males utilise health resources more than females [22].
A study of different age groups among HIV patients revealed that the maximum number of patients in stage 3 13 (10.83%) patients and stage 2 10 (8.3%) patients belonged to the age group of 36 to 45 years, whereas the maximum number of patients in stage 4 12 (10%) patients belonged to the age group of 46 to 55 years and stage 1 9 (7.5%) patients belonged to the age group of 26 to 35 years. Age is a major risk factor for neurocognitive disorders in patients living with HIV. Studies have shown that the aging of the HIV population contributes to the development of cognitive impairment among older individuals [28,29]. In a systematic review and meta-analysis conducted by Zenebe Y et al., five studies identified older age as a significant risk factor for HAND. A pooled analysis of these studies revealed an odds ratio of 3.68 (95% CI: 2.95, 4.11), indicating a strong association between advancing age and the likelihood of developing neurocognitive impairments in individuals living with HIV [30].
In the present study, more than half of the study population (53%) had no co-morbidities. Among the remaining patients, diabetes mellitus (19.2%) was the most common co-morbidity recorded, followed by hypertension 15 (12.5%) patients and healed pulmonary tuberculosis 6 (5%) patients. In a study conducted by Nlooto M involving 516 cases of HIV, hypertension was the most common co-morbidity present in 39% of cases, followed by diabetes mellitus in 12%, which aligns with our study’s findings [31]. HIV infection, along with multiple physiological and psychosocial factors, is known to cause neurocognitive disorders. Co-morbidities such as cardiovascular disease, diabetes, sleep disorders and co-infection with hepatitis C may contribute to the development of HAND [28].
Out of the 120 patients in the present study, 19%, 25%, 35% and 21% belonged to WHO clinical stages 1, 2, 3 and 4, respectively. Among the 21 patients with higher CD4 counts of >500, 90% were in stage 1. In contrast, out of the 31 patients with lower CD4 counts of <100, 58% were classified as having WHO stage 4 disease. This indicates that a lower CD4 count is associated with a higher stage of the disease, which increases the risk of opportunistic infections and AIDS-defining illnesses. The CD4+ T-lymphocyte count is the most widely used measure of HIV-induced damage to the immune system. In a study conducted by Edathodu J et al., involving 191 patients with HIV, a significant correlation was found between CD4 count and WHO stage of the disease [32]. The severity of the disease increases as the WHO clinical stage progresses from 1 to 4, with patients in clinical stage 4 being at greater risk.
Patients with higher CD4 counts tended to exhibit less neurocognitive impairment, suggesting a lower burden of disease. It has also been reported that HIV-infected individuals who consistently maintain higher CD4 cell counts are relatively protected from neurocognitive impairment, whereas patients with a history of severe immunosuppression are found to experience more neurocognitive challenges. This may be attributed to the fact that lower levels of CD4 T-cells may allow a larger number of viruses to enter the central nervous system [11,33-36].
The calculated prevalence of ANI, MND and HAD in the current study was 29%, 35% and 36%, respectively. The association between patients’ CD4 count and the Frascati classification was found to be statistically significant (p<0.05). In a study conducted by Gandhi NS et al., among 104 patients with HIV, 36% had ANI, 21% had MND and 31% had HAD, which is similar to our findings [37]. Patients with lower CD4 counts exhibited neurocognitive impairment and higher staging according to the Frascati criteria.
In a systematic review and meta-analysis conducted by Zenebe Y et al., advanced clinical stages of HIV and a CD4 count of 500 cells/μL or lower are the most frequently reported factors associated with an increased risk of HAND [30]. Globally, the prevalence of HAND is observed to be approximately 50% among all HIV patients. The prevalence of HAND has been reported to be as high as 85% [38,39] and as low as less than 10% [22]. In this study, 51% of patients with HIV disease were found to be neurocognitively impaired when tested using the 3MS. This result aligns with a study conducted in Chennai and Bengaluru, India, which reported the prevalence to be between 50% and 60% [40]. In the current study, the association between patient counts and classifications based on 3MS scores was found to be statistically significant (p<0.05). A study conducted by Kumar S et al., found that patients’ CD4 counts are strongly associated with 3MS scores [22].
Among the 3MS scores, 15 cognitive domains were tested, including date and place of birth, registration, mental reversal, first recall, temporal orientation, spatial orientation, naming, four-legged animal, similarities, repetition, read and obey, writing, copying two pentagons, three-stage commands and second recall. It was observed that all parameters were below normal values. The greatest decrease was seen in the similarities category (46%), followed by mental reversal (43%), read and obey (42%), first recall (41%) and second recall (40%). It was learned that certain domains of cognitive functioning—such as motor functioning, attention, processing speed, executive functioning, learning, verbal memory, reasoning and verbal fluency—are often affected in patients suffering from HIV disease due to cognitive deficits [9,41]. The present study aligns with the results reported by Kumar S et al., where parameters such as second recall, repetition, copying two pentagons, read and obey, mental reversal and first recall were found to be most affected in patients with HIV disease [22].
A systematic review has highlighted the role of cognitive assessment tools, such as the 3MS, in detecting cognitive impairments in individuals living with HIV, even during the era of HAART. This underscores the continued relevance of cognitive testing in identifying early signs of HAND and the ongoing need for effective management strategies in clinical settings [42]. Screening for HAND is a simple and inexpensive bedside test. This study examined the association between cognitive function in HAART-naïve patients using the 3MS and the relationship between the 3MS scores and the immunological status of HAART-naïve patients.
Additionally, recent studies have expanded the exploration of biomarkers and additional diagnostic methods in conjunction with the 3MS to improve the accuracy of HAND detection [43-45]. These developments emphasise the role of the 3MS in recognising cognitive impairments in individuals living with HIV and highlight the need for ongoing research in clinical environments to refine and enhance diagnostic approaches.
Limitation(s)
While these findings are promising, the present study has certain limitations. Long-term follow-up data are necessary for the generalisability of the current findings. Larger, randomised controlled trials are needed to validate these results.
Conclusion(s)
The HAND is an important cause of morbidity. With the availability of highly effective ART, there has been a reduction in severe forms of HAND worldwide. However, milder forms of HAND persist and remain a clinical concern for PLHIV. The current study has shown a prevalence of HAND at 50.83%, which is in agreement with the global prevalence reported. The CD4 count of the patients was found to be strongly associated with neurocognitive disorders in the present study. Patients with higher CD4 counts exhibited less neurocognitive impairment. A shorter version of the modified Mini-mental Examination, with parameters such as the similarities category, mental reversal, read and obey, first recall and second recall, which were found to be affected in the patients in the present study, would be useful in effectively screening for HAND.
*Men who have sex with men
Chi-square value-29.21; p value-0.001; *ANI: Asymptomatic neurocognitive impairment; +HAD: HIV-associated dementia; #MND: Mild neurocognitive disorder
COVID-19: Coronavirus disease-2019