The COVID-19 pandemic emerged in December 2019 and millions of deaths are attributed to it. India contributed significantly to morbidity and mortality associated with COVID-19 [1]. The COVID-19 pandemic has swept India in three waves [2]. The first wave of the pandemic peaked in mid-September 2020 with almost 1,00,000 cases per day [3]. The second wave began in the end of February 2021 ushering with it a fateful surge of COVID-19 cases largely attributed to the Delta variant of the virus. The third wave of the pandemic began at the end of December 2021 and diminished by March 2022 [3-5].
COVID-19 vaccination in India was rolled out in January 2021 using a need-based approach starting with the healthcare workers, followed by frontline workers. The second phase of vaccination catered to the senior citizens (above 60 years of age), individuals with co-morbidities and aged >45 years and later to individuals aged 45-59 years. In the third phase, vaccination was administered to individuals aged 18-44 years [4,6]. This was followed by the vaccination of children aged 15 to 18 years with Covaxin and subsequently of children from 12 to 14 years with the CorBEvax vaccine [7,8]. Initially vaccination was administered with a schedule of two doses separated by four weeks. As evidence suggested better protection by increasing the interval between the two doses, the government of India issued a notification for the administration of the second dose of the vaccine between six to eight weeks after the first dose. This interval was further prolonged to 12-16 weeks in May 2021 [9].
Though the disease is no longer a public health emergency, with every passing time newer variants of COVID-19 are emerging and the possibility of another wave with a new variant cannot be disregarded. The constantly evolving nature of the pandemic necessitates on-going studies related to disease characteristics and vaccine efficacy. Geographic variations have been observed in the epidemiology of COVID-19, with some countries often with advanced healthcare facilities experiencing higher morbidity and mortality compared to the developing countries [18]. This study intended to estimate the association of COVID-19 positivity with COVID-19 vaccination status among the subjects presenting for testing at our Diagnostic Molecular Laboratory and was conducted with the following objectives:
Materials and Methods
An electronic record-based cross-sectional study was conducted from April 2021 to September 2021 in the Department of Microbiology, Shridevi Institute of Medical Sciences and Research Hospital, Tumkur, Karnataka, India, after obtaining approval from the Institutional Ethics Committee (ECR/831/Inst/KA/2016/RR-19). The clinicodemographic data from participants were collected and entered by trained sample collection staff on the RT-PCR mobile application (RT-PCR) [19] developed by the National Informatics Centre, Ministry of Electronics and Information Technology, Government of India. The details were then extracted from the data entry portal hosted by the Indian Council of Medical Research (ICMR COVID-19 Data Portal) [20] for COVID-19 testing as a single dataset. The dataset was anonymised before use. The study adhered to the tenets of the Declaration of Helsinki.
Inclusion criteria: Data of all participants aged ≥18 years, whose nasopharyngeal and/or oropharyngeal samples were received at the diagnostic molecular laboratory for COVID-19 testing either by RT-PCR or RAT, during the study period (April 2021 to September 2021) were included in the study.
Exclusion criteria: Participants with incomplete data were excluded from the study.
At the time of the study, COVID-19 testing was done for various reasons as per the ICMR guidelines [21]. The study population included the following:
a) Symptomatic patients with Severe Acute Respiratory Illness (SARI) or Influenza Like Illness (ILI);
b) Asymptomatic high-risk patients (immunocompromised, postorgan transplant, co-existing co-morbidities, elderly ≥65 years seeking hospitalisation), patients undergoing invasive procedures and pregnant women hospitalised for delivery;
c) Individuals undertaking international travel or travel to Indian states requiring COVID-19 test at the point of entry;
d) Individuals who wish to voluntarily get themselves tested.
The period of sample collection was divided into two halves. The first half was from April 2021 to June 2021, which included the phase of the peak of the second wave of the pandemic and the second half was from July 2021 to September 2021 during which period India saw a steady decline in COVID-19 positivity [6].
Data collection and case definition: Data pertaining to patient demographics, presence or absence of symptoms, presence of co-morbidities like diabetes mellitus, hypertension, etc., and vaccination status were collected.
Samples that amplified both the ORF1 ab and the N gene on RT-PCR for SARS CoV-2 (Meril COVID-19 One-step RT-PCR Kit, Meril Diagnostics Pvt., Ltd., India) or showed a coloured band for both test and control on RAT (STANDARD Q® COVID-19 Antigen kit, SD Biosensor Healthcare Pvt. Ltd., India) were considered positive for SARS CoV-2.
Vaccination status: Patients were considered as partially or fully vaccinated after 14 days of receiving 1st or 2nd dose of the COVID-19 vaccine, respectively. Patients not satisfying the above criteria were classified as unvaccinated [22]. At the time of conducting the study, only two doses of the vaccine were recommended by the Ministry of Health and Family Welfare in India for the prevention of COVID-19 illness [6].
Statistical Analysis
Descriptive variables of patient characteristics were summarised using suitable measures of central tendencies for continuous data (means and medians), variability {Standard Deviation (SD) and Interquartile Range (IQR)}, and frequencies or percentages for categorical data. The Chi-square test was used to assess the association between categorical variables. A bivariate analysis was performed using logistic regression to identify factors that were associated with COVID-19 positivity. Multivariate logistic regression was used to assess the relationship between variables after adjusting for potential confounders. A non parsimonious model approach was used to include all the independent variables for multivariate analysis irrespective of the strength of association on bivariable analysis. Spearman correlation coefficient was calculated to assess the relationship between vaccination status and COVID-19 positivity. Data was analysed using Stata 17 software (StataCorp. 2021. Stata Statistical Software: Release 17. College Station, TX, USA: StataCorp LLC.). The statistical significance level for all outcomes was considered at two-sided alpha of 0.05 and 95% confidence intervals were presented for all effect estimates.
Results
A total of 14,853 patients aged ≥18 years were tested for COVID-19 during the study period. Seven samples were excluded due to incomplete data and a total of 14,846 participants were included in the study. More than half of the samples were obtained in the month of May 2021 (n=7847, 52.9%) with a gradual decline in the samples in the second half of the study.
[Table/Fig-1] shows the demographic characteristics of the study population. The mean age of the participants was 40.2±16.2 years (range: 18-101 years) with a male preponderance, male to female ratio being 1.3:1. About one-fifth of the samples tested positive for COVID-19 (n=3380; 22.8%). About 466 (3.1%) patients had at least one co-morbidity, the commonest being Diabetes mellitus in 322 (69.1%) followed by hypertension in 184 (39.48%). The other co-morbidities 147 (31.54%) seen were cardiac disease, renal disease, lung disease, and malignancy. About 1/4th (n=3380) of the patients tested had symptoms suggestive of COVID-19 and 13.54% (n=2010) had received at least one dose of the COVID-19 vaccine.
Demographic characteristics of study population.
| Characteristic | N=14846 |
|---|
| Age (Mean±SD) (years) | 40.2±16.2 |
| Gender (n, %) | Male | 8370 (56.4) |
| Female | 6476 (43.6) |
| COVID-19 status (n, %) | Positive | 3,380 (22.8) |
| Negative | 11,466 (77.2) |
| Co-morbidities (n, %) | No co-morbidity | 14,380 (96.9) |
| Presence of at least one co-morbidity | 466 (3.1) |
| Symptoms suggestive of COVID-19 (n, %) | Symptomatic | 3,818 (25.7) |
| Asymptomatic | 11,028 (74.3) |
| Vaccination type (n, %) | Unvaccinated | 12,836 (86.46) |
| Covishield | 1,807 (12.17) |
| Covaxin | 199 (1.34) |
| Sputnik V | 4 (0.03) |
Samples that were tested in the second half (July to September 2021) of the study had 87% lower odds of testing positive for COVID-19 (OR: 0.13, 95% CI: 0.10-0.16, p-value <0.001) compared to those tested in the first half of the study. This difference was found to be significant even after adjusting for vaccination status (OR: 0.16, 95% CI: 0.13-0.2, p-value <0.001). After adjusting for age, gender, co-morbidity and period of testing, the odds of COVID positivity reduced by 32% (AOR: 0.68; 95% CI: 0.56 to 0.82; p-value <0.001) with one dose of COVID-19 vaccine and reduced by 59% (AOR: 0.41; 95% CI: 0.31 to 0.53; p-value <0.001) with two doses of COVID-19 vaccine compared to no vaccination. COVID-19 positivity among vaccinated patients was found to be 9.75% (n=196). The vaccination rate among the COVID-19 positive patients was found to be significantly lower than the COVID negative patients (5.8% vs. 15.82%, p-value <0.001). COVID-19 positivity was found to be significantly lower among fully vaccinated in comparison to the partially vaccinated individuals (6.32% Vs 12.92%, p-value <0.001). COVID-19 positivity decreased from 24.81% (n=3184) in unvaccinated individuals to 12.92% (n=135) in individuals who had received only one dose of the vaccine. In individuals who had received two doses of the vaccine COVID-19 positivity further reduced to 6.32% (n=61) [Table/Fig-2].
Vaccination status and COVID-19 positivity among those presenting for COVID-19 testing at a tertiary care centre (n=14,846).
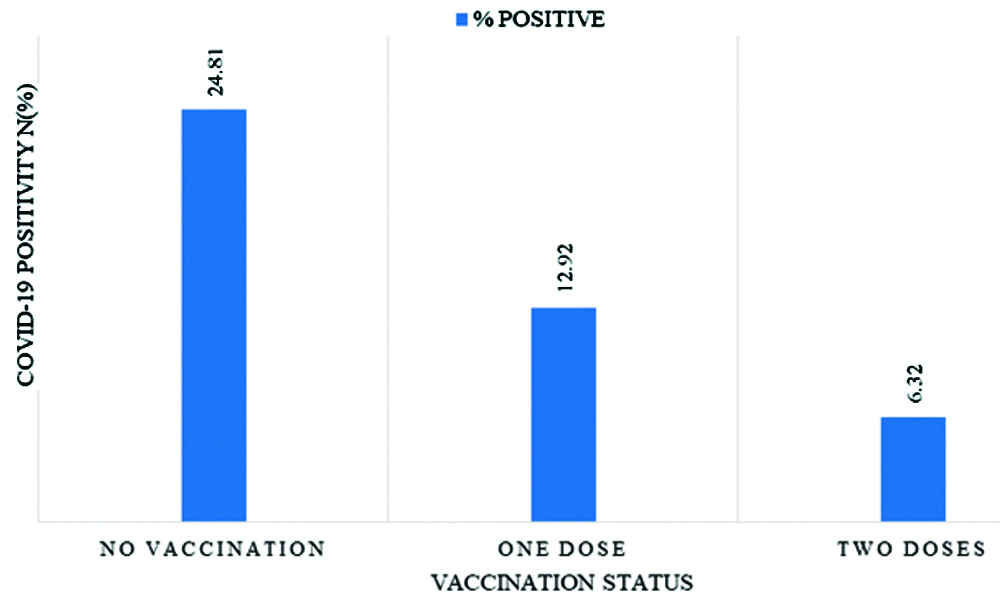
Female gender and vaccination against COVID-19 were found to be associated with 22% (Crude OR: 0.78, 95% CI: 0.72-0.85, p-value <0.001) and 67% (Crude OR: 0.33, 95% CI:0.28-0.38; p<0.001) lower risk of association with COVID-19 positivity [Table/Fig-3]. Bivariate analysis of COVID positivity among vaccinated and unvaccinated individuals revealed a 55% reduced odds of testing COVID-19 positive after a single dose of the COVID-19 vaccine (OR: 0.45, 95% CI: 0.37-0.54; p-value <0.001). The odds of testing positive for COVID-19 further reduced to 80% with receipt of two doses of the vaccine (OR: 0.20, 95% CI: 0.16-0.27; p-value <0.001). Multivariate analysis after adjusting for the effects of age, gender and time of pandemic, showed that receiving a single dose of COVID-19 vaccine reduced the odds of having a COVID-19 positive status by 32% (AOR: 0.68, 95% CI: 0.56-0.82; p-value <0.001) and receiving two doses of the vaccine lowered the odds of turning COVID-19 positive by 59% (AOR: 0.41, 95% CI: 0.31-0.53; p-value <0.001).
Association between characteristics of participants and COVID-19 positivity in a tertiary care hospital in India (n=14,846).
| Parameter | COVID-19 positive (n=3380) | COVID-19 negative (n=11466) | Crude odds ratio (95% CI) | p-value |
|---|
| Age (Mean±SD) | 39.9±16.4 | 41.4±15.5 | 1.01 (1.003-1.008) | <0.001 |
| Gender (n, %) | Male | 2062 (24.64) | 6308 (75.36) | 1.0 | <0.001 |
| Female | 1318 (20.35) | 5158 (79.65) | 0.78 (0.72-0.85) |
| Vaccination status (n, %) | Not vaccinated | 3184 (24.81) | 9652 (75.19) | 1.0 | <0.001 |
| Vaccinated | 196 (9.75) | 1814 (90.25) | 0.33 (0.28-0.38) |
No of vaccine doses
(n, %) | Nil | 3184 (24.81) | 9652 (75.19) | 1.00 | |
| 1st dose | 135 (12.92) | 910 (87.08) | 0.45 (0.37-0.54) | <0.001 |
| 2nd dose | 61 (6.32) | 904 (93.68) | 0.20 (0.16-0.27) | <0.001 |
| Co-morbidity* (n, %) | Absent | 3278 (22.8) | 11102 (77.2) | 1.0 | |
| Present | 102 (21.89) | 364 (78.11) | 0.95 (0.76 -1.19) | 0.65 |
| Period of sample collection (n, %) | April-June, 2021 | 3291 (25.81) | 9462 (74.19) | 1.0 | <0.001 |
| July-September, 2021 | 89 (4.25) | 2004 (95.75) | 0.13 (0.10-0.16) |
*Presence of at least one co-morbidity
Since the independent variable (age) is a continuous variable, there was no baseline variable and was interpreted as the odds of increase of COVID-19 positivity with every unit increase in age (for every one year of increase in age). With one year increase in age the odds of COVID-19 positivity increases by 1%.
A weak negative correlation was found between COVID-19 positivity and vaccination status (Spearman’s Rhoρs=-0.1209, p-value <0.01). With decreased rate of vaccination in the first half of the study, the rate of COVID positivity was found to be 25.81% (n=329). There was a notable decrease in the COVID-19 positivity in the second half of the study to 4.25% (n=89) with increase in the vaccination rates to 49.45%. [Table/Fig-4] shows the trend of COVID-19 positivity and COVID-19 vaccination status from April to September 2021.
COVID vaccination and positivity trend April-September 2021.

COVID-19 positivity among patients with co-morbidity was found to be 21.89% (n=103) and in those without co-morbidity was 22.80% (n=3278). On multivariate analysis after adjusting for gender, age, vaccination status and period of sample collection, having at least one co-morbid condition increased the odds of COVID-19 positivity by 28% (AOR: 1.28, 95% CI 1.01-1.63, p-value=0.039) [Table/Fig-5].
Multivariate analysis after adjusting for various clinicodemographic variables among participants presenting for COVID-19 testing.
| Parameters | Adjusted Odds Ratio (AOR) (95% CI) | p-value |
|---|
| Age | 1.00 (1.00 to 1.00) | 0.189 |
| Gender | Male | 1.00 | <0.001 |
| Female | 0.85 (0.79 to 0.93) |
| Vaccination status | Not vaccinated | 1.00 | |
| Vaccinated | 0.55 (0.44 to 0.68) | <0.001 |
| No. of vaccine doses | Not vaccinated | 1.00 | |
| Vaccinated-1st dose | 0.68 (0.56 to 0.82) | <0.001 |
| Vaccinated-2nd dose | 0.41 (0.31 to 0.53) | <0.001 |
| Co-morbidity* | Absent | 1.00 | 0.039 |
| Present | 1.28 (1.01 to 1.63) |
| Sample collection period | April to June 2021 | 1.00 | <0.001 |
| July to September 2021 | 0.16 (0.13 to 0.20) |
*Presence of atleast one co-morbidity
Discussion
The current study underscores the importance of vaccination in reducing COVID-19 positivity. COVID-19 positivity rate in present study was found to be 22.8%. COVID-19 positivity was found to be significantly higher in male gender. This difference was significant even after adjusting for age, vaccination status, presence of co-morbidities and time of sample collection. Previous studies have also recorded a significant male preponderance in the reported cases and deaths [2,4].
Patients who submitted the samples in the second half of the study had 87% lower odds of testing positive. A study by Mandal S et al., that used mathematical models of infectious disease transmission demonstrated that quarantining 50% of symptomatic individuals can reduce the cumulative incidence by 62% and the peak prevalence by 89% [23]. A nation-wide survey demonstrated that two-thirds of the general population aged ≥6 years and 85% of healthcare workers were sero-positive for SARS Co-V-2 by June-July 2021 in India irrespective of vaccination status which can be attributed to natural infection [24]. A study conducted by Murali AJ et al., on healthcare workers exposed to COVID-19 patients demonstrated a secondary infection rate of 2.07% (CI 0.7-3.4%) [25]. The decline in positivity rate in the latter half of the study could be attributed to increased vaccination coverage and natural infection.
The presence of co-morbidities was associated with a higher risk of COVID-19 infection. The most common co-morbidity found in present study patients was diabetes mellitus followed by hypertension which was consistent with findings of Tendulkar P et al., [2]. However, a systematic review and meta-analysis estimating the prevalence of co-morbidities in COVID-19 patients reported that hypertension was the commonest co-morbidity accounting for 21% of the cases followed by diabetes mellitus (11%) [26]. Similar observations were made in a meta-analysis that studied publications on Indian populations [27]. It was found in present study that presence of even a single co-morbid condition increased the odds of COVID-19 positivity by 28%. However, the effect of co-morbidities on disease outcome was not assessed due to lack of follow-up of cases. COVID-19 patients requiring hospitalisation have been found to have a higher prevalence of co-morbidities compared to the general population [28].
In present study, 13.54% of the patients were vaccinated against SARS-CoV-2. Vaccination rate gradually increased from 7.68% in the first half of the study to almost 50% in the second half. A study conducted by Vanathy K et al., demonstrated the utility of the social media campaign in creating awareness about the importance of COVID-19 vaccination [29]. Vaccination coverage was found to be significantly higher in the female gender. Vaccination rate among patients testing positive for COVID-19 was significantly lower. Further a weak negative correlation was found between rate of COVID-19 positivity and vaccination status over the duration of the study. A notable decrease in the COVID-19 positivity was observed in the second half of the study with a corresponding increase in vaccination coverage. Multivariate analysis revealed that there was a general decline in COVID-19 positivity in the second half of the study irrespective of vaccination status.
However, further multivariate analysis after adjusting for the effects of age, gender and time of pandemic, showed that receiving a single dose of COVID-19 vaccine reduced the odds of having a COVID-19 positive status by 32% and receiving two doses of the vaccine lowered the odds of turning COVID-19 positive by 59% irrespective of phase of sample collection. This emphasises the role of vaccination as an independent factor contributing to reduction in odds of COVID-19 positivity. de Gier B et al., compared individuals who had hybrid immunisation against SARS CoV-2 (vaccination and prior infection) against vaccine induced immunity alone. Their study concluded that in individuals with equal number of prior immunising events, hybrid immunity provided 71-85% lower risk of infection with omicron variant for 4-10 weeks after the last event depending on number of prior immunisations [30].
In a multicentric study conducted in India on the effectiveness of Covaxin and Covishield vaccines, partial vaccination was found to be 65% effective and complete vaccination was 83% effective against severe COVID-19 disease [31]. A study conducted by Sinha P et al., demonstrated that neutralising antibodies were detected in 61.89% of the participants four weeks after the first dose and 84.3% of the participants four weeks after the second dose of the Covaxin vaccine [32].
Bernal L et al., demonstrated that a single dose of Pfizer-BioNTech vaccine is about 60-70% effective and with two doses the efficacy increases to 85-90% [33]. Moghadas SM et al., suggested an approximately 50% reduction in infection following vaccination with a mean overall attack rate of 4.6% among the vaccinated in comparison to 9% in the unvaccinated group [34].
Patel AK et al., demonstrated that the unvaccinated patients had a lower Ct (Cycle threshold) value in RT-PCR suggesting a higher viral load in the respiratory sample [35]. Similarly, Levine-Tiefenbrun M et al., demonstrated that the Ct value in individuals who tested positive 12-37 days after vaccination, was significantly higher than the unvaccinated control group. Thus, vaccinated people can be considered potentially less infectious and vaccination could thus play a role in preventing the transmission of the disease [36]. In present study, around 10% of the vaccinated individuals were found to be COVID-19 positive. These breakthrough infections in vaccinated individuals are speculated to a lack of an adequate immune response to the vaccine or a subsequent infection with a different variant [37].
Limitation(s)
The current study aimed at determining an association between vaccination status and COVID-19 positivity. The findings of present study could have been confounded by factors like subclinical infections, pre-existing immunity due to infection with related coronaviruses, and infection prevention and control measures like habit of wearing mask, maintaining social distancing which were not assessed in present study. Effectiveness of the vaccines in preventing severe disease and effect of co-morbidity on disease outcome was not assessed due to lack of follow-up of the cases. Hence the relationships are only associations and causality cannot be implicated due to the cross-sectional nature of the study.
Conclusion(s)
Present study showed significantly decreased odds of COVID-19 positivity in vaccinated individuals. The reduction in odds of testing positive for SARS-CoV-2 was found to be dose dependent, with individuals receiving two doses of the vaccine showing lower odds of COVID-19 positivity versus individuals receiving one dose. Though the COVID-19 pandemic is at the cusp of endemicity with the risk of emergence of new variants, constant surveillance and further studies aimed at understanding the role of the vaccine in preventing infection and disease transmission will better equip us for future challenges.
*Presence of at least one co-morbidity
*Presence of atleast one co-morbidity