The WHO defines the rational use of medicine as the provision of medication that aligns with the patient’s clinical needs, administered at the correct dosage, for the appropriate period and at the lowest cost affordable to the patient [1,2]. Hence, cost consideration by the prescriber is an important criterion, along with other criteria of rational prescription, while achieving an affordable therapeutic cure. India is known as the “pharmacy of the world” [3]. The Indian pharmaceutical industry encompasses around 3,000 drug companies and more than 10,500 manufacturing units [4]. There are over 60,000 generic brands across 60 therapeutic categories available [5]. Indians consume Rs. 56,000 crore in medicines through private pharmacists [6]. The second leading cause of rural debt in India is healthcare, after dowry [7,8]. Although not universal, the Medical Council of India (MCI) recommends that doctors write generic prescriptions [9]. The prescribing doctor expects a certain treatment outcome. The doctor must protect the patient by prescribing an affordable, good-quality generic or brand that matches their needs [10]. Otherwise, therapy may fail. Failure to comply due to the cost of an unaffordable brand could result in antibiotic resistance or failure to treat infectious diseases [11].
Fluoroquinolones are among the most valuable and widely used antibiotics. About 30% of the global pharmaceutical market consists of fluoroquinolones. Levofloxacin and ciprofloxacin account for 65% ($3.3 billion) of fluoroquinolone sales [12]. The National Pharmaceutical Pricing Authority (NPPA) of India has imposed a ceiling on drug costs via the DPCO to regulate soaring drug prices. Pharmaceutical companies can only price drugs below the ceiling set. The 2024 DPCO list includes levofloxacin, ciprofloxacin, moxifloxacin and ofloxacin. JAK is an Indian government program that provides affordable generic medications, helping to make healthcare more affordable in India [13].
Materials and Methods
A cross-sectional descriptive analytical study was carried out between December 2022 and August 2024 to provide a perspective on the latest information across the two-year study period. Authors from different locations in India collaborated to use commercial drug directories for the study. The research was conducted at Command Hospital (Air Force) in Bengaluru, Karnataka, India. Ethical committee clearance was obtained (IEC Certificate No: CHAFB/IEC/77/2024) for the study.
Data collection: The costs of oral fluoroquinolones, both as single medications and in combinations, of the same strength and dosage form manufactured by different companies were obtained from the CIMS for April to July 2022 [21] and “Drug Today” for April to July 2022, along with the latest CIMS for April to July 2024 and “Indian Drug Review" for May and June 2024. All of these are authentic commercial drug directories [22-24]. The Bureau of Pharma PSUs of India (BPPI) websites were reviewed for generic drug pricing to compare branded and generic prices [25]. This study utilises JAK for generic medication costs. The ceiling costs of fluoroquinolone tablets were taken from the NPPA list for April 2022 and May 2024 and compared to the maximum prices after extrapolating for 10 tablets [26,27]. The difference between the maximum and minimum prices of drug formulations manufactured by different pharmaceutical companies was calculated, along with the cost ratio and percentage of cost variation [17].
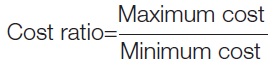
Percentage of cost variation was calculated as follows:

Statistical Analysis
A simple descriptive analysis was performed on all the data collected, which was entered into Microsoft 365 (Version 2407) Excel spreadsheets.
Results
[Table/Fig-1] illustrates the cost variation of oral fluoroquinolones in INR (Indian Rupees), cost ratio and percentage variation in cost from 2022 to 2024. Of the 25 drug formulations, levofloxacin 500 mg exhibited the greatest variance, at 11,048.64% in 2022 and 2,080.18% in 2024, with the cost for 10 tablets being Rs. 8.80 (2022) and Rs. 45.00 (2024) and Rs. 981.08, respectively. From 2022 to 2024, this broad fluctuation improved dramatically (11,048.64% vs. 2,080.18%). Although the variation is still considerable, it indicates a false positive change towards the improvement of variation. The minimum cost for levofloxacin has increased from Rs. 8.80 to Rs. 45.00, while the maximum price has remained the same. Hence, despite the percentage variation decreasing from 11,048.64% to 2,080.18%, this does not represent a true improvement. Only 4 of the 25 formulations had less than 25% cost variance in 2022 and none of the formulations in 2024 had a variance of less than 25%. The cost ratio and percentage variation are improving; however, the overall scenario remains comparable.
Variation of cost of single drug therapy of fluoroquinolones in 2022 and 2024.
| S. No. | Drug and dose (mg) | In the year 2022 | In the year 2024 |
|---|
| Minimum cost/10 tablets (INR) 2022 | Maximum cost/10 tablets (INR) 2022 | Cost ratio 2022 | % Variation in cost 2022 | Minimum cost/10 tablets (INR) 2024 | Maximum cost/10 tablets (INR) 2024 | Cost ratio 2024 | % Variation in cost 2024 | Change in % variation |
|---|
| 1 | Balofloxacin 100 | 83 | 140 | 1.69 | 68.67 | 83.5 | 140 | 1.68 | 67.66 | -1.01 |
| 2 | Ciprofloxacin 100 | 12.52 | 25.2 | 2.01 | 101.28 | 12.75 | 25.2 | 1.98 | 97.65 | -3.63 |
| Ciprofloxacin 250 | 12.5 | 131.05 | 10.48 | 948.40 | 12.5 | 131.05 | 10.48 | 948.40 | 0.00 |
| Ciprofloxacin 500 | 20 | 137.5 | 6.88 | 587.50 | 16.87 | 112.28 | 6.66 | 565.56 | -21.94 |
| Ciprofloxacin 750 | 59.51 | 308.65 | 5.19 | 418.65 | 55 | 142.94 | 2.60 | 159.89 | -258.76 |
| Ciprofloxacin 1000 | 120 | 120 | 1.00 | 0.00 | 85.06 | 186 | 2.19 | 118.67 | 118.67 |
| 3 | Gemifloxacin 320 | 100 | 718 | 7.18 | 618.00 | 100 | 580 | 5.80 | 480.00 | -138.00 |
| 4 | Levofloxacin 250 | 23 | 59 | 2.57 | 156.52 | 37.5 | 75 | 2.00 | 100.00 | -56.52 |
| Levofloxacin 500 | 8.8 | 981.08 | 111.49 | 11048.64 | 45 | 981.08 | 21.80 | 2080.18 | -8968.46 |
| Levofloxacin 750 | 11 | 132.5 | 12.05 | 1104.55 | 76 | 135 | 1.78 | 77.63 | -1026.91 |
| 5 | Lomefloxacin 400 | 90 | 185 | 2.06 | 105.56 | 106 | 185 | 1.75 | 74.53 | -31.03 |
| 6 | Moxifloxacin 400 | 196.9 | 800 | 4.06 | 306.30 | 196.9 | 800 | 4.06 | 306.30 | 0.00 |
| 7 | Norfloxacin 100 | 13.5 | 17 | 1.26 | 25.93 | 13.25 | 18.8 | 1.42 | 41.89 | 15.96 |
| Norfloxacin 200 | 23.4 | 39 | 1.67 | 66.67 | 5.57 | 44.72 | 8.03 | 702.87 | 636.21 |
| Norfloxacin 400 | 10.71 | 68 | 6.35 | 534.92 | 10.4 | 68 | 6.54 | 553.85 | 18.93 |
| Norfloxacin 800 | 41.06 | 97.5 | 2.37 | 137.46 | NA | NA | NA | NA | - |
| 8 | Ofloxacin 100 | 20 | 50.6 | 2.53 | 153.00 | 20 | 61.05 | 3.05 | 205.25 | 52.25 |
| Ofloxacin 200 | 24.95 | 80.52 | 3.23 | 222.73 | 30 | 88.5 | 2.95 | 195.00 | -27.73 |
| Ofloxacin 400 | 48.95 | 594.28 | 12.14 | 1114.06 | 62.4 | 594.28 | 9.52 | 852.37 | -261.68 |
| 9 | Pefloxacin 400 | 24.06 | 49.5 | 2.06 | 105.74 | 24.06 | 49.5 | 2.06 | 105.74 | 0.00 |
| 10 | Prulifloxacin 600 | 240 | 792.7 | 3.30 | 230.29 | NA | NA | NA | NA | - |
| 11 | Sprafloxacin 100 | 28.76 | 60.3 | 2.10 | 109.67 | 28.76 | 82.47 | 2.87 | 186.75 | 77.09 |
| Sprafloxacin 200 | 48.1 | 250 | 5.20 | 419.75 | 30.85 | 150 | 4.86 | 386.22 | -33.53 |
| Sprafloxacin 300 | 150 | 150 | 1.00 | 0.00 | NA | NA | NA | NA | - |
| Sprafloxacin 400 | 150 | 173.4 | 1.16 | 15.60 | 153.86 | 153.86 | 1.00 | 0.00 | -15.60 |
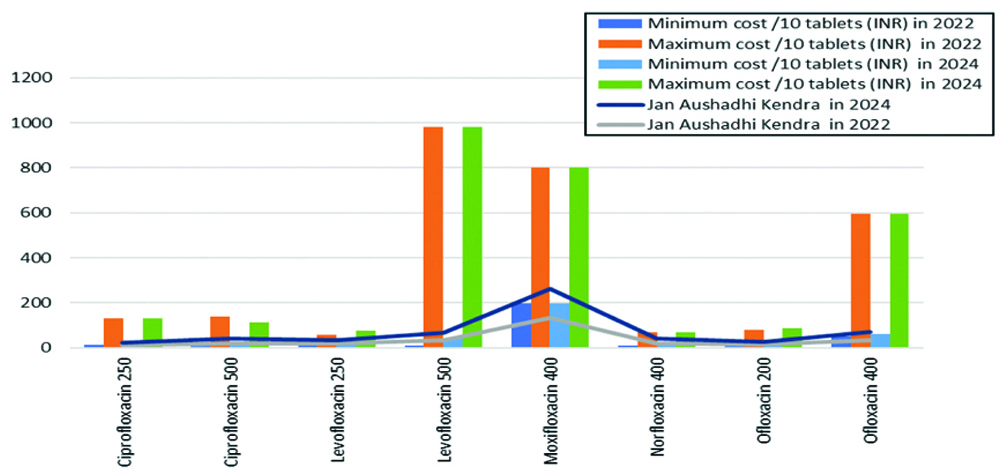
[Table/Fig-2,3] compares JAK generic prices to the minimum and maximum costs for 10 tablets of each formulation. In 2022, five JAK (generic) formulations cost less than their branded counterparts, one was equally priced and two were more expensive. In 2024, JAK generics of six formulations cost less than the branded versions, while two branded formulations were cheaper than their generic counterparts. This shows that generics were cheaper than the minimum branded prices for ciprofloxacin 250 mg, levofloxacin 250 mg, moxifloxacin 400 mg, ofloxacin 200 mg and ofloxacin 400 mg in both 2022 and 2024. However, in 2022, the price of ciprofloxacin 500 mg was equal to the minimum branded price and in 2024, the generic was not cheaper than the minimum branded price. For levofloxacin 500 mg, the generic was not cheaper than the branded version in 2022, but it was cheaper in 2024, whereas for norfloxacin 400 mg, the situation was reversed.
Cost variation of single drug between brand (Minimum and Maximum) vs generics.
| S. No. | Drug and dose (mg) | In the year 2022 | In the year 2024 |
|---|
| Minimum cost/10 tablets (INR) | Maximum cost/10 tablets (INR) | Jan Aushadhi Kendra (JAK) | Generic cheaper than branded | Minimum cost/10 tablets (INR) | Maximum cost/10 tablets (INR) | Jan Aushadhi Kendra (JAK) | Generic cheaper than branded |
|---|
| 1 | Ciprofloxacin 250 | 12.5 | 131.05 | 11 | Yes | 12.50 | 131.05 | 11 | Yes |
| 2 | Ciprofloxacin 500 | 20 | 137.5 | 20 | Equal | 16.87 | 112.28 | 20 | No |
| 3 | Levofloxacin 250 | 23 | 59 | 18 | Yes | 37.50 | 75.00 | 18 | Yes |
| 4 | Levofloxacin 500 | 8.8 | 981.08 | 33 | No | 45.00 | 981.08 | 33 | Yes |
| 5 | Moxifloxacin 400 | 196.9 | 800 | 132 | Yes | 196.90 | 800.00 | 132 | Yes |
| 6 | Norfloxacin 400 | 10.71 | 68 | 21 | No | 10.40 | 68.00 | 21 | No |
| 7 | Ofloxacin 200 | 24.95 | 80.52 | 14 | Yes | 30.00 | 88.50 | 14 | Yes |
| 8 | Ofloxacin 400 | 48.95 | 594.28 | 35 | Yes | 62.40 | 594.28 | 35 | Yes |
Cost variation of fluoroquinolone Brands (min vs max) vs generic in single drug.
[Table/Fig-4] displays the minimum and maximum costs per 10 tablets in INR, cost ratio and percentage cost variation of oral fluoroquinolone combinations in various formulations. Twenty-five formulations were noted. In 2022, norfloxacin 400 mg and tinidazole 600 mg had the highest percentage variation (3,233.33%), followed by ofloxacin 200 mg and ornidazole 500 mg with 2,364.29%. In 2024, the highest percentage cost variation was observed for ofloxacin 200 mg and ornidazole 500 mg, at 1,029.73%, followed by norfloxacin 400 mg and tinidazole 600 mg. In 2022, 16 of the 25 formulations showed more than 100% variation. The percentage difference is improving and there are numerous combinations that are currently unavailable.
Variation of cost of combinations drugs of Fluoroquinolones in 2022 and 2024.
| S. No. | Drug combination dose (mg) | In the year 2022 | In the year 2024 | |
|---|
| Minimum cost/10 tablets (INR) | Maximum cost/10 tablets (INR) | Cost ratio | % Variation in cost | Minimum cost/10 tablets (INR) | Maximum cost/10 tablets (INR) | Cost ratio | % Variation in cost | % decrease in variation |
|---|
| 1 | Ciprofloxacin+Ornidazole 500+500 | 92.40 | 105.80 | 1.15 | 14.50 | 105.80 | 105.80 | 1.00 | 0.00 | 100.00 ↓ |
| 2 | Ciprofloxacin+Tinidazole 250+300 | 35.82 | 72.50 | 2.02 | 102.40* | 31.08 | 79.67 | 2.56 | 156.34* | -52.68 ← |
| 3 | Ciprofloxacin+Tinidazole 500+600 | 22.80 | 155.96 | 6.84 | 584.04* | 25.00 | 155.96 | 6.24 | 523.84* | 10.31 ↓ |
| 4 | Norfloxacin+Tinidazole 400+600 | 3.15 | 105.00 | 33.33 | 3233.33* | 15.30 | 105.00 | 6.86 | 586.27* | 81.87 ↓ |
| 5 | Norfloxacin+Ornidazole 400+500 | 50.00 | 50.00 | 1.00 | 0.00 | NA | NA | NA | NA | NA |
| 6 | Norfloxacin+Nitazoxanide 400+500 | 99.00 | 99.00 | 1.00 | 0.00 | NA | NA | NA | NA | NA |
| 7 | Norfloxacin+Tinidazole+Probiotic 400+600+Varies | 97.71 | 97.71 | 1.00 | 0.00 | 60.00 | 97.71 | 1.63 | 62.85 | - |
| 8 | Norfloxacin+Metronidazole+Probiotic 400+500+Varies | 67.00 | 67.00 | 1.00 | 0.00 | NA | NA | NA | NA | NA |
| 9 | Ofloxacin+Probiotics 200+Varies | 42.00 | 60.00 | 1.43 | 42.86 | NA | NA | NA | NA | NA |
| 10 | Ofloxacin+Probiotics 400+Varies | 80.00 | 80.00 | 1.00 | 0.00 | NA | NA | NA | NA | NA |
| 11 | Ofloxacin+Tinidazole 100+300 | 50.00 | 167.00 | 3.34 | 234.00* | NA | NA | NA | NA | NA |
| 12 | Ofloxacin+Tinidazole 200+600 | 39.00 | 143.00 | 3.67 | 266.67* | 48.00 | 143.00 | 2.98 | 197.92* | 25.78 ↓ |
| 13 | Ofloxacin+Tinidazole 200+300 | 58.07 | 162.50 | 2.80 | 179.83* | 162.50 | 162.50 | 1.00 | 0.00 | 100.00 ↓ |
| 14 | Ofloxacin+Tinidazole 200+500 | 69.68 | 69.68 | 1.00 | 0.00 | NA | NA | NA | NA | NA |
| 15 | Ofloxacin+Tinidazole 300+600 | 62.50 | 62.50 | 1.00 | 0.00 | NA | NA | NA | NA | NA |
| 16 | Ofloxacin+Tinidazole 400+600 | 97.45 | 97.45 | 1.00 | 0.00 | 97.45 | 97.45 | 1.00 | 0.00 | NA |
| 17 | Ofloxacin+Ornidazole 200+500 | 7.00 | 172.50 | 24.64 | 2364.29* | 18.50 | 209.00 | 11.30 | 1029.73* | 56.45 ↓ |
| 18 | Ofloxacin+Ornidazole 100+250 | 53.00 | 53.00 | 1.00 | 0.00 | NA | NA | NA | NA | NA |
| 19 | Ofloxacin+Ornidazole+Probiotics 200+500 | 72.64 | 1180.00 | 16.24 | 1524.45* | 108.00 | 119.90 | 1.11 | 11.02 | 99.28 ↓ |
| 20 | Ofloxacin+Nitazoxanide 200+500 | 89.00 | 117.70 | 1.32 | 32.25 | 89.00 | 117.70 | 1.32 | 32.25 | 0.00 |
| 21 | Levofloxacin+Ornidazole 250+500 | 65.00 | 107.88 | 1.66 | 65.97 | 72.18 | 113.00 | 1.57 | 56.55 | 14.28 ↓ |
| 22 | Levofloxacin+Ornidazole 500+500 | 89.50 | 89.50 | 1.00 | 0.00 | 80.00 | 89.50 | 1.12 | 11.88 | NA |
| 23 | Levofloxacin+Azithromycin 250+250 | 128.70 | 176.00 | 1.37 | 36.75 | 128.70 | 199.00 | 1.55 | 54.62 | -48.63 0 |
| 24 | Levofloxacin+Azithromycin 500+500 | 228.50 | 290.40 | 1.27 | 27.09 | 228.50 | 290.40 | 1.27 | 27.09 | 0.00 |
| 25 | Norfloxacin+Probiotics 500+Varies | 89.00 | 89.00 | 1.00 | 0.00 | 17.80 | 70.97 | 3.99 | 298.71* | NA |
[Table/Fig-5] compares JAK generics to the minimum and maximum costs of 10 pills for branded formulations of oral fluoroquinolone combinations. In both 2022 and 2024, only one generic formulation was priced lower than the minimum cost of the branded formulations. One generic formulation, namely moxifloxacin 400 mg and cefixime 400 mg, was exclusively available through JAK and was not listed in drug formularies; hence, it could not be compared.
Comparison of Fluoroquinolone drug combinations available at Jan Aushadhi Kendra (JAK) with minimum and maximum costs per 10 tablets.
| S. No. | Drug combination | Dose (mg) | In the year 2022 | In the year 2024 |
|---|
| Minimum cost/10 Tablets (INR) | Maximum cost/10 Tablets (INR) | Generic drug (Jan Aushadhi Kendra) (JAK) | Generic cheaper than branded | Minimum cost/10 Tablets (INR) | Maximum cost/10 Tablets (INR) | Generic drug (Jan Aushadhi Kendra) (JAK) | Generic cheaper than branded |
|---|
| 1. | Ciprofloxacin+Tinidazole | 250+300 | 35.82 | 72.50 | 28.00 | Yes | 31.08 | 79.67 | 28 | Yes |
| 2. | Ciprofloxacin+Tinidazole | 500+600 | 22.8 | 155.96 | 39.00 | No | 25.00 | 155.96 | 39 | No |
| 3. | Ofloxacin+Ornidazole | 200+500 | 7.00 | 172.5 | 27.50 | No | 18.50 | 209.00 | 27.50 | No |
| 4. | Norfloxacin+Tinidazole | 400+600 | 3.15 | 105.00 | 40.00 | No | 15.30 | 105.00 | 40 | No |
| 5. | Moxifloxacin+Cefixime | 400+400 | NA | NA | 170.00 | NA | NA | NA | 170 | NA |
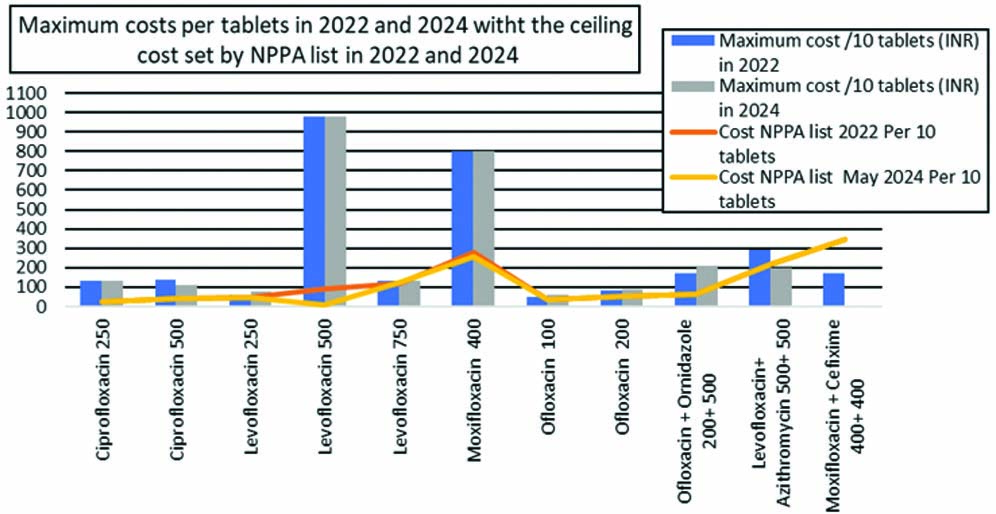
[Table/Fig-6] compares oral fluoroquinolone formulations on the NPPA list for April 2022 and May 2024, detailing the maximum cost per 10 pills and ceilings in rupees. The cost of all formulations, except for moxifloxacin 400 mg and cefixime 400 mg, listed by the NPPA in April 2022 exceeded the ceiling pricing, as they were not included in the drug formularies in 2024.
Comparison of maximum of costs of oral fluoroquinolones in 2022 and 2024 with ceiling prices issued by NPPA list April 2022 and May 2024.
| S. No. | Drug | Dose (mg) | In the year 2022 | | In the year 2024 | |
|---|
| Maximum cost/10 tablets (INR) | NPPA list April 2022 per tablet | Cost NPPA list 2022 per 10 tablets | Max cost more than NPPA ceiling cost | Maximum cost/10 tablets (INR) | Cost NPPA list May 2024 per tablets | NPPA list May 2024 per 10 tablets | Max cost more than NPPA Ceiling cost |
|---|
| 1. | Ciprofloxacin | 250 | 131.05 | 2.30 | 23.0 | Yes | 131.05 | 2.2 | 22 | Yes |
| 500 | 137.50 | 4.05 | 40.5 | Yes | 112.28 | 4.25 | 42.5 | Yes |
| 2. | Levofloxacin | 250 | 59.00 | 4.90 | 49.0 | Yes | 75.00 | 4.82 | 48.2 | Yes |
| 500 | 981.08 | 8.95 | 89.5 | Yes | 981.08 | 8.99 | 8.99 | Yes |
| 750 | 132.50 | 12.14 | 121.4 | Yes | 135.00 | 12.45 | 124.5 | Yes |
| 3. | Moxifloxacin | 400 | 800 | 28.13 | 281.3 | Yes | 800.00 | 25.82 | 258.2 | Yes |
| 4. | Ofloxacin | 100 | 50.6 | 3.55 | 35.5 | Yes | 61.05 | 3.55 | 35.5 | Yes |
| 200 | 80.52 | 5.26 | 52.6 | Yes | 88.50 | 5.26 | 52.6 | Yes |
| 5. | Ofloxacin+Ornidazole | 200+500 | 172.5 | 6.6 | 66.0 | Yes | 209 | 6.6 | 66.0 | Yes |
| 6. | Levofloxacin+Azithromycin | 500+500 | 290.4 | 21.77 | 217.7 | Yes | 199 | 21.77 | 217.7 | Yes |
| 7. | Moxifloxacin+Cefixime | 400+400 | 170.00 | 34.46 | 344.6 | No | NA | 34.46 | 344.6 | NA |
[Table/Fig-7] illustrates the comparison of cost variation for oral fluoroquinolones included in the NPPA list for April 2022 and May 2024, along with their ceiling prices, in a visual manner.
Comparison of cost variation of oral Fluoroquinolones included in NPPA list April 2022 and May 2024 with its ceiling prices.
Discussion
In India, different pharmaceutical companies, including the manufacturers, sell the same drug under various brand names. This results in a single formulation being available under different brand names and at different prices. Few studies have examined the cost variance of medication formulations available in the market. The significant cost variance of medications has puzzled prescribing clinicians, who presume that cheaper formulations may lack pharmacological efficacy [28] and may fail to treat disorders [29]. Therapy failure can lead to patient distrust of the clinician and to health consequences that could have been prevented if the medication were not counterfeit. In 2021, many pharmaceuticals failed to meet FDA quality standards, resulting in the cancellation of licenses for 46 Indian pharmaceutical companies [30]. Patients may lose wages and incur out-of-pocket costs if they opt for a more expensive brand. High drug costs can lead to non compliance, therapeutic failure and antibiotic resistance, especially in the case of fluoroquinolones.
According to Article 21 of India’s Constitution, every individual has the right to health care [31]. The Indian government has consistently provided this right. The DPCO governs drug prices in India. The government issues the DPCO under Section 3 of the Essential Commodities Act, 1955, to set and regulate the prices of essential bulk drugs and their formulations. The NPPA regulates the prices of medicines in India [13]. It periodically adjusts the prices of controlled bulk medications and formulations. The NPPA also recovers overcharges and regulates the pricing of decontrolled medicines.
The recent NPPA list allows pharmaceutical companies to set prices below the ceiling [4]. As a result, these medicines should be sold at lower prices. The latest NPPA list was issued in May 2024 and on April 22, prior to present study analysis. Present study investigation indicated that all formulations with NPPA list ceiling prices from April 2022 had prices that exceeded the CIMS and IDR ceiling prices from April to July 2022. A similar situation was observed in 2024.
Despite efforts to control drug pricing, certain studies have found considerable cost disparities for various pharmaceuticals of pharmacoeconomic relevance in India. Hetawal P et al., found substantial cost variations for fluoroquinolones in their analysis conducted in 2021 and 2022 [18]. A similar trend was observed in present study. Six of the seven fluoroquinolones on the NPPA list in April 2022 (10 out of 11 formulations) had prices above the ceiling. This was also true for moxifloxacin 400 mg and cefixime 400 mg, which were exclusively available through JAK, a government initiative. Upon reassessment in 2024, present analysis found no changes in this situation. No studies were found comparing fluoroquinolone cost variation to NPPA list prices.
Present study discovered a notable improvement in price variation from 2022 to 2024; however, the cost variation was still far from achieving the dream of affordability and the expected ceiling prices set by the NPPA. Chawan VS et al., observed a similar issue in 2015 in their research titled “Fluoroquinolones in India: Are We Prescribing It Right? A Cost Variation Study” [17]. Dhanvijay PV and Manwatkar SK obtained similar results in 2020 [19]. This situation is concerning in India, the “pharmacy of the world” and a developing nation. India did an excellent job of distributing COVID-19 vaccinations internationally at a low price, demonstrating that Indians can afford high-quality, non spurious drugs.
The promotion of generic pharmaceuticals is an effective alternative strategy to lower patient medication costs. Generic medications are claimed to be the same as and bioequivalent to brand-name drugs in terms of dosage, intended use, effects, side-effects, route of administration, risks, safety and strength. However, the efficacy and bioequivalence of generic drugs have been questioned in comparison to branded drugs. In 2008, India launched the Jan Aushadhi Scheme to provide cheaper generic medications to the public. Jan Aushadhi generics are reported to have comparable therapeutic efficacy to branded medications. However, there is a common misconception that generic medications are always inexpensive. Present study observations indicated that not all JAK formulations were the cheapest. Hetawal P et al., and Atal S et al., found similar results [18,32].
Prescribers’ skepticism regarding the efficacy of generic drugs must be addressed [33,34]. Regular quality assurance studies can determine the exact drug content in the claimed product and formulation drug content analyses are essential to rule out counterfeit pharmaceuticals. This approach will help make safe, inexpensive and effective pharmaceuticals accessible to everyone.
Incorporating pharmacoeconomics as a practical lesson in undergraduate and postgraduate medical curricula, where students compute the cost of their prescriptions, may assist them in understanding its implications and fostering the necessary mindset. Information about bioequivalence should be included in CIMS and IDR books, along with cost data. Increasing public awareness of this significant price range will help healthcare providers, payers, government agencies, policymakers and pharmacists to collaborate and take action.
Limitation(s)
This analysis utilised ready reckoner drug formulary pricing. Since these are Maximum Retail Prices (MRPs) and do not include pharmacy discounts or exemptions, the actual market rates at which these pharmaceuticals are supplied to consumers remain unknown. Additionally, the materials may lack certain drug brands.
Conclusion(s)
There is a wide cost variation in the formulations of oral fluoroquinolones in India in the years 2022 and 2024. The costs of all the formulations of oral fluoroquinolones listed in the NPPA list of April 2022 and the NPPA list of May 2024 were not within the set ceiling prices. Although improvements have been observed, we are still far from the goal of affordable medication for all, as outlined by the ceiling prices set by the NPPA. There is a need to sensitise all stakeholders to achieve the vision of safe, affordable and effective medications for everyone. Future studies should be conducted at regular intervals to assess the situation and any improvements in the cost variation of this essential medicine, fluoroquinolone, to ensure that healthcare is accessible and affordable to those in need.