Case Report
Case 1
A 61-year-old female came to the hospital with the complaint of shortness of breath for two months, loss of appetite and loss of weight of 4 kg in the last three months. The shortness of breath was grade 3-4 according to the modified Medical Research Council (mMRC) dyspnoea scale, which was relieved by aspirating the fluid from the right side of the chest, done prior to admission at our institution. However, the patient was a non smoker and had no history of cough, expectoration, fever, chest pain or any significant comorbidities. The patient gave a history of bronchial asthma in her mother. On examination, her vitals and systemic examination were normal. High-Resolution Computerised Tomography (HRCT) showed right pleural effusion with collapse and consolidation of the right lung, multiple nodules in the right upper lobe and mediastinal lymphadenopathy. Initial blood investigations revealed normal cell counts and renal and liver function tests were within normal range. Chest X-ray showed right hydropneumothorax which was aspirated. The pleural fluid culture showed no growth, and cytological analysis was negative for malignant cells and GeneXpert showed no detection.
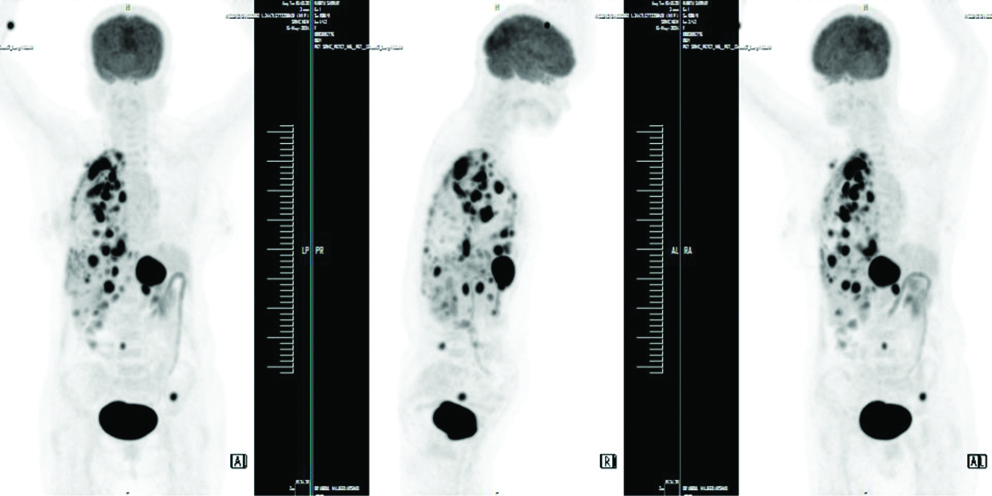
The patient underwent a 2-D echocardiogram which showed normal chambers, an ejection fraction of 63% with normal pulmonary artery pressure. PET/CT scan showed FDG avid collapse consolidation seen involving the whole of the right middle lobe and most of the anterobasal segment of the right lower lobe. Also, a relatively well-defined enhancing FDG avid lesion (Standardised Uptake Value [SUVmax] 11.9) in the collapsed lateral segment of the right middle lobe was seen, measuring approximately 4.3×2.4×2.5 mm with metastasis to the right pleura, lymph nodes, pleural deposits, liver, adrenal gland, right L5 vertebral pedicle and left iliac bone [Table/Fig-1,2 and 3].
Positron Emission Tomography-Computerised Tomography (PET-CT) scan: Well-defined enhancing Fluorodeoxyglucose (FDG) avid lesion seen (SUVmax 11.9) in collapsed lateral segment of right middle lobe, measuring approximately 4.3×2.4×2.5 mm - likely malignant lesion.
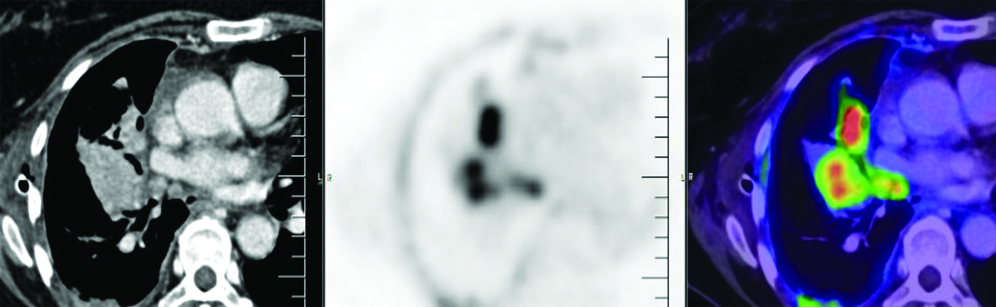
PET/CT scan: Multiple FDG avid (SUVmax 16.1) thick enhancing areas of pleural thickening seen involving most of the right pleura and fissures, the maximum thickness measuring approximately 19 mm—likely pleural metastasis.
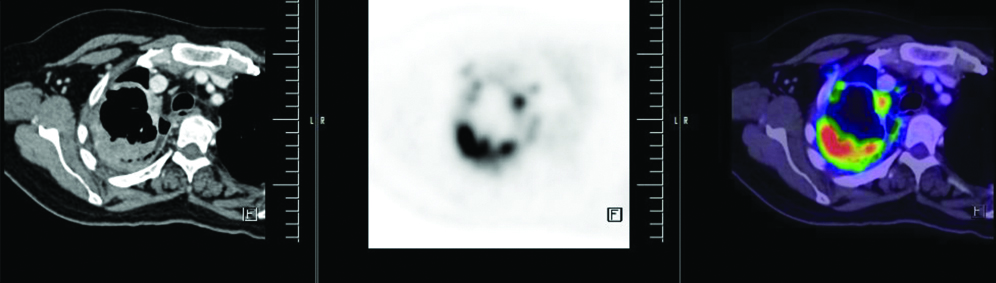
PET/CT scan: Well-defined malignant lesion the right middle lobe of lung with metastatic lesions in the right pleura, multiple lymph nodes, right anterior extra pleural, liver, left adrenal gland, right L5 vertebral pedicle and left iliac bone.
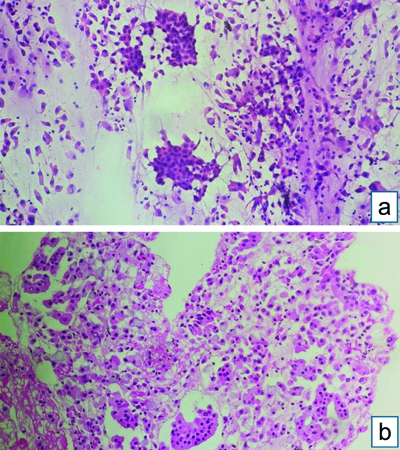
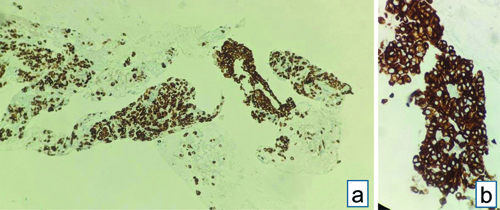
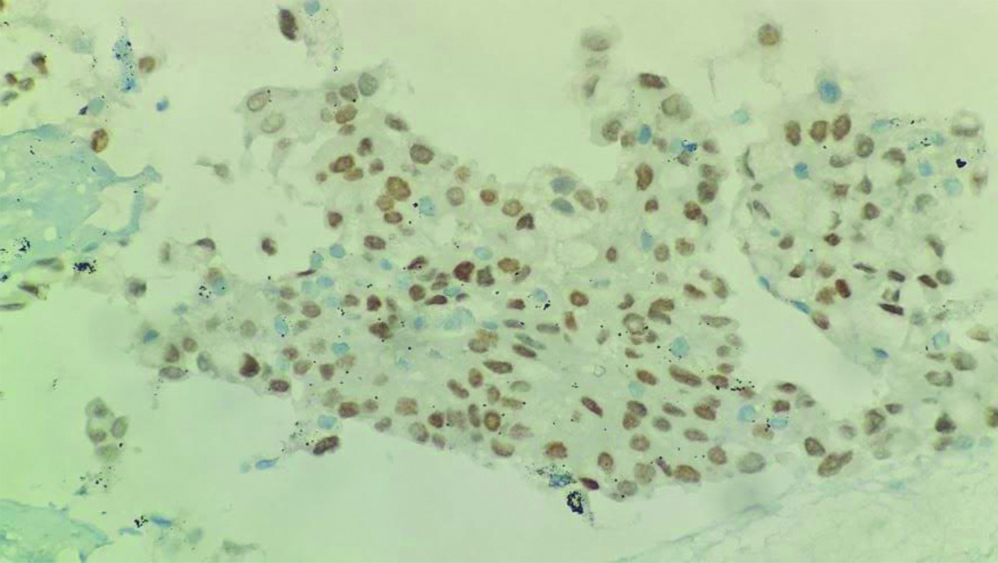
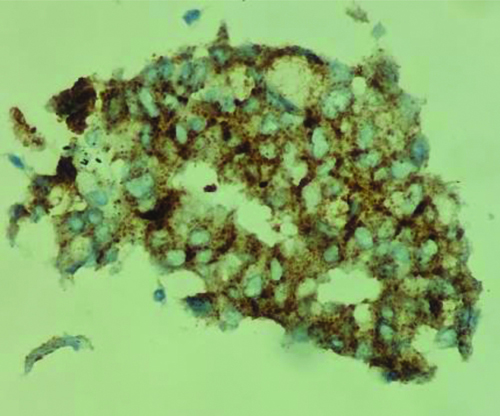
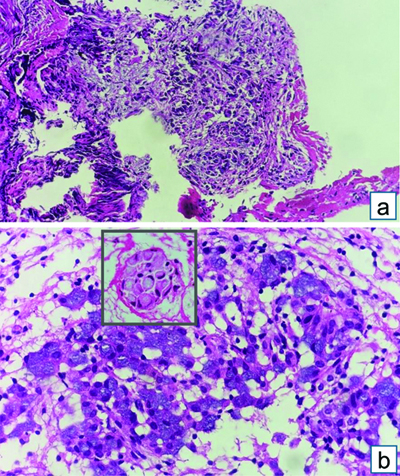
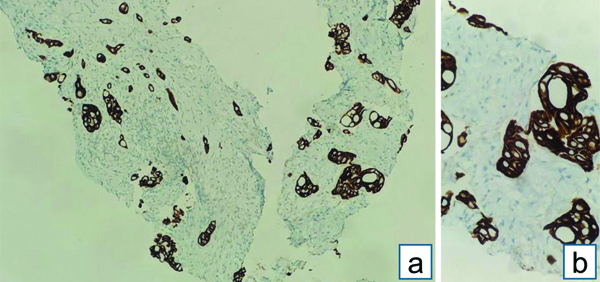
Endobronchial Ultrasound-guided Transbronchial Needle Aspiration (EBUS-TBNA) with a total of 7 passes was done. The aspirate and bronchial wash were reported as suspicious for malignant cells [Table/Fig-4a]. The EBUS-TBNA core [Table/Fig-4b] and endobronchial biopsies were received as multiple very tiny grey-white to grey-brown soft-tissue bits altogether aggregating to 0.5 cc. Histopathological analysis of the endobronchial biopsy [Table/Fig-5] revealed the diagnosis of lung adenocarcinoma with SRC morphology characterised by abundant intracellular mucin accumulation and a crescentic nucleus displaced toward one end of the cell. This was confirmed by IHC, the tumour cells showed positivity for markers CK7 [Table/Fig-6] and TTF-1 [Table/Fig-7]. Further, the tumour was positive for ALK [Table/Fig-8] gene rearrangements and negative for ROS proto-oncogene1 (ROS1) fusion by IHC. Epidermal Growth Factor Receptor (EGFR) mutational analysis was done by Polymerase Chain Reaction (PCR) and no mutation of the EGFR gene was detected.
EBUS-TBNA: (a) Aspirate showing cellular smear, atypical cells with mild to moderate nuclear pleomorphism, admixed with bronchial epithelial cells- Suspicious of malignancy (Haematoxylin and Eosin [H&E] stain x100); (b) Core biopsy showing atypical cell clusters, few with intracytoplasmic mucin, along with bronchial epithelial cell nests (H&E stain x100).
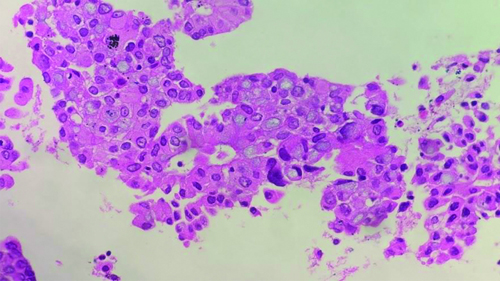
Endobronchial biopsy showing atypical cell clusters, few with intracytoplasmic mucin, resembling Signet Ring Cells (SRC), along with bronchial epithelial cells nests (H&E stain x200).
CK7 IHC: Strong diffuse membranous positivity in tumour cells. (a) (IHC x100); (b) (IHC x400).
TTF-1 IHC: Diffuse strong nuclear positivity in tumour cells (IHC x200).
ALK IHC: strong granular cytoplasmic positivity in tumour cells (IHC x400).
Computed Tomography Pulmonary Angiography (CTPA) was done to rule out pulmonary thromboembolism as the patient continued to be hypoxic and had developed haemoptysis and persistent tachycardia, requiring oxygen therapy. CTPA showed right lower lobe loculated effusion, for which thoracocentesis was attempted with only a dry tap. This was followed-up with the medical thoracoscopic procedure which revealed multiple nodules over the costal surface of the pleura and diaphragm with dense adhesions. Partial adhesiolysis was done and multiple pleural biopsies were taken, which were positive for malignancy, suggestive of adenocarcinoma with SRC morphology [Table/Fig-9].
Pleural biopsy: (a) Fibrocollagenous tissue with infiltration by nests and vague glands of tumour cells (H&E stain x100); (b) Vague glands (inset) nest of tumour cells exhibiting intracellular mucin simulating Signet Ring Cells (SRC) (H&E stain 10x) (Inset-H&E stain 40x).
Currently, the patient is receiving Crizotinib, a first-generation ALK inhibitor, approved for first-line treatment in patients with advanced and metastatic ALK-positive Non Small Cell Lung Cancer (NSCLC). She has been advised regular CT or PET scans every 2-3 months to assess the response to therapy and monitor for disease progression, along with liver function tests and electrolytes studies during treatment, and to be vigilant for other symptoms like rashes, diarrhoea, etc.
Case 2
A 62-year-old female presented with complaints of breathlessness, cough with expectoration, and loss of weight for the past six months, which was gradually progressive. She was a known diabetic and hypertensive for 15 years, on irregular treatment. There was no significant family history. Physical examination revealed an enlarged palpable lymph node of 1.7×1.5 cm in size in the left supraclavicular region with moderate mobility. Her vitals and systemic examination were normal. Chest X-ray showed mild left pleural effusion. Biochemical investigations proceeded and her liver function tests were mildly elevated with levels of ALT 61 U/L, Aspartate Transaminase (AST) 45 U/L, Alkaline Phosphatase (ALP) 152 U/L, Gamma-Glutamyltransferase (GGT) 39 U/L, total bilirubin 2 mg/dL, albumin, 7.8 g/L, total proteins 13.8 g/dL and Prothrombin Time (PT) of 16 seconds. PET/CT done outside showed FDG avid lesion (SUVmax 14.9) measuring 2.3×1.6×1 cm seen involving the left lower lobe, favouring lung carcinoma, with multiple enlarged lymph nodes. Non FDG avid lesions seen in the breast were also reported, which could be indicative of a benign breast lesion.
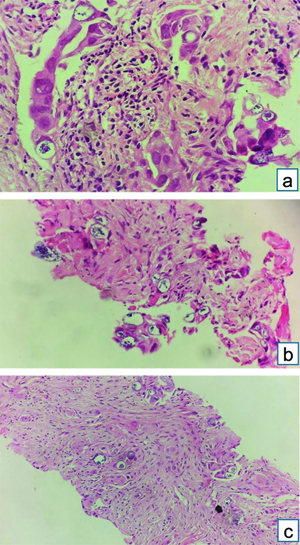
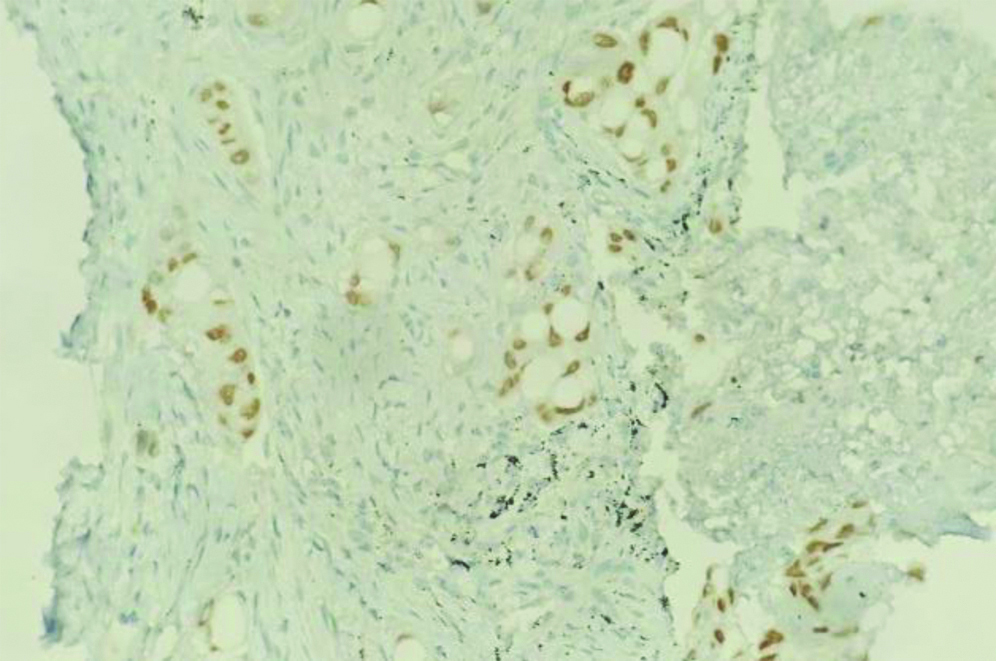
CT-guided biopsy of the mass was done and sent for histopathological analysis, where multiple grey-white linear cores of tissue ranging in size from 0.2 to 0.6 cm were received. A diagnosis of lung adenocarcinoma with SRC morphology (primary/secondary was made) [Table/Fig-10]. Further analysis with IHC was done, with tumour cells showing positivity of CK7 [Table/Fig-11] and TTF-1 [Table/Fig-12], while not expressing CK20 and GATA-3. Hence, a diagnosis of primary adenocarcinoma of lung with SRC morphology was made.
CT-guided biopsy of lung mass: (a) Linear core of tissue with small nests of tumour cells and few singled-out cells (H&E stain x100); (b) Cords and vague glands of round to oval tumour cells with moderate eosinophilic cytoplasm and moderate nuclear pleomorphism, along with few mucin-filled cells (H&E stain x400); (c) Singled-out tumour cells with intracytoplasmic mucin and eccentrically placed nucleus, resembling SRCs (H&E stain x200).
CK7 IHC: Strong diffuse membranous positivity in tumour cells. (a) (IHC x100); (b) (IHC x400).
TTF-1 IHC: Diffuse moderate nuclear positivity in tumour cells (IHC x200).
The patient was advised to undergo molecular testing for EGFR, KRAS mutations, and ALK rearrangements, which were done outside. The tumour was positive for EGFR exon-19 deletion. The patient underwent lobectomy following which she was put onOsimertinib, a third-generation EGFR Tyrosine Kinase Inhibitor (TKI). However, she was advised close regular monitoring of liver function and to be aware of any side effects of the TKI.
Discussion
Worldwide, lung cancer is the third most common malignancy, with significant mortality and morbidity rates. According to GLOBOCAN 2020, lung cancers contributed to about 14.3% of new cases in males, followed closely by prostatic cancer [1]. NSCLC constitutes about 85% of all lung cancers. NSCLCs are typically subdivided into adenocarcinoma, squamous Cell Carcinoma (SqCC), and large-cell carcinoma [2]. Adenocarcinoma is the most predominant histological type of lung cancer. They generally occur in the periphery of the lung; however central location is not uncommon. Adenocarcinoma has a predilection to females especially female smokers, thence contributing to recent increasing trends compared to SqCC. Over the past 50 years, changes in the design and composition of cigarettes have resulted in smokers taking deeper inhalations which is believed to increase the exposure of peripheral airway cells to carcinogens, the primary site of impact. Additional risk factors include exposure to radon gas, second-hand tobacco smoke, indoor pollutants, and environmental pollution [3]. Primary lung adenocarcinoma with SRC morphology is a rare entity, which was originally described by Kish JK et al., and lung adenocarcinoma with SRC was considered to be a unique subtype of lung cancer in the 2004 WHO lung adenocarcinoma classification, which was designated as SRC adenocarcinoma [4,5]. However, in the 2015 WHO lung cancer classification, lung adenocarcinoma with SRCs is viewed as a cytologic change that may occur in association with various histological patterns rather than as a separate subtype [6].
Because of the rarity of SRC in primary tumours of the lung, metastatic disease is often considered in the differential diagnosis, IHC is helpful if histology is inconclusive [7]. Primary pulmonary adenocarcinomas with SRC have been associated with ALK gene rearrangement. However, the incidence of ROS1 gene fusions in lung adenocarcinomas with SRC is not well known [7]. Herein reported are two cases of this rare entity reported in one-month duration, characterising the cytomorphological and molecular features. In addition, we also evaluated the genetic abnormalities in these tumours including EGFR, ALK and ROS1.
SRCs are characterised by abundant intracellular mucin accumulation and a peripherally displaced crescentic nucleus. Carcinomas with SRCs may arise in various organs, especially the stomach. Other sites include colon, urinary bladder, prostate, and breast [8]. Metastasis from these sites must be ruled out with radiological correlation, owing to its more common occurrence before making this rare diagnosis of primary lung adenocarcinoma with SRC morphology. The treatment and expected prognosis are usually dependent on the identification of the primary site of cancer. Immunohistochemical studies can be useful in suggesting an origin. The expression pattern of CK20 and CK7 immunohistochemical markers may be particularly helpful in this regard. In addition, TTF-1 is commonly expressed in carcinomas of the thyroid and lung and studies have indicated that this regulatory nuclear protein could serve as a marker for distinguishing these tumours from morphologically similar malignancies arising in other organs [9]. In a study by Merchant SH et al., 82.4% of the 17 pulmonary SRCCs exhibited TTF-1 positivity. However, none of the non pulmonary SRCCs was positive for TTF-1 [9]. CDX2, SATB2 and villin were used as markers to distinguish between adenocarcinoma of the colon from pulmonary adenocarcinoma. Few studies, however, showed a less specific nature of villin. Testing for oestrogen and Progesterone Receptors (PRs) expression in metastatic SRCCs of unknown primary can be useful as these receptors can be potential targets for anti-hormonal agents, with respect to metastatic SRCCs of breast, which usually don’t express E-cadherin, like metastatic gastric SRCCs [10,11]. In these patients with cancer of unknown primary, furthermore, Molecular Tumour Profiling (MTP) can help in predicting the tissue of origin [10]. In the WHO Classification of lung tumours 2004 edition, signet ring lung adenocarcinoma was considered a unique subtype of lung cancer [6]. However, in the 2015 and the latest 2021 WHO Classifications, SRC morphology is viewed as a cytological change that may occur in certain unique genetic alterations [12].
Primary pulmonary adenocarcinoma with SRC morphology is relatively rare, with an aggressive clinical course, metastasises to lymph nodes, predominantly seen in non smokers, and harbours ALK gene rearrangements that may benefit ALK tyrosine-kinase inhibitor therapies [7,8].
In the Vallonthaiel AG et al., study [13], only 11 cases showed the presence of SRCs out of 218 pulmonary adenocarcinomas, of which seven had paired cytology and histopathology samples available and the remaining four cases had only histopathology samples. In our study, one case had EBUS-TBNA aspirate as well as core biopsy samples. Vallonthaeil AG et al., expressed the 2 cytomorphological variants of the SRC, cells with a single large intracytoplasmic mucin vacuole along with an eccentric nucleus, (Single mucin vacuole/SMV type) [13]. The other morphological type was described as cells with histiocyte-like finely vacuolated cytoplasm, round nucleus and prominent nucleoli, resembling alveolar macrophages (Histiocyte-like/HL type). Both our cases showed abundant mucin vacuole. Tsuta K et al., classified the tumours with SRC components as H-SRCC and L-SRCC based on the percentage of SRC component in the tumours (<50% and >50%, respectively) [14]. Other studies classified adenocarcinomas with SRC morphology based on other components present, for example, Chen C et al., divided their cases into two groups based on whether they contained solid components [15].
It is vital to establish a definitive diagnosis based on histologic examination however, immunohistochemical staining is often helpful, as primary pulmonary adenocarcinomas with SRCs frequently express TTF-1 and CK7 and are usually negative for CK20 and CDX-2. In the present case, the tumour cells were diffusely immune-positive for TTF-1 and CK7.
ALK expression was seen on IHC, whereas ROS1 was negative, similar to the report by Muscarella LA et al., who confirmed both the protein expression by Fluorescence In Situ Hybridisation (FISH) and performed Next-Generation Sequencing (NGS) analysis which revealed the presence of the neuregulin 1 gene (NRG1) fusion transcript variant CD 74 molecule gene (CD74) ex6–NRG1 ex6 [16]. TTF-1 has a decisive role as a master regulatory transcription factor in lung development and maintenance of functions of Terminal Respiratory Unit (TRU) cells, and a strong positive relationship between TTF-1 protein expression and ALK rearrangement has been observed in previous studies [17]. In Boland JM et al., study, ROS1 fusion gene positivity by FISH was seen in only three of 47 (6%) SRC pulmonary adenocarcinoma cases tested [7]. However, 14 cases (26%) showed ALK rearrangement (ALK+) by FISH. Ha SY et al., study of the 16 ALK-positive adenocarcinomas, 13 showed mucinous background [18]. SRC features were seen in 14 of the 16 cases, (87.5%; p<0.001). Common histological patterns in this study included sheets with tubulopapillary or tubulocribriform patterns. In the study by Tsuta K et al., SRCC components were seen in just 39 cases (1.5%) of 2640 cases of surgically resected primary lung carcinomas, of which 36 cases were part of an adenocarcinoma [14]. In carcinomas where the SRCC component occupied ≥50% of the lesion, the 5-year survival rates were low, with the frequencies of lymphovascular invasion, and lymph node metastasis being significantly higher than in non SRCC carcinomas. Nishino M et al., studied the ALK gene rearrangement using dual-color break apart probe specific to the ALK locus on chromosome 2p23, and a tumour was considered positive for ALK rearrangement if 15% of cells showed split signals. Of the 54 ALK+ cases, 39 showed SRCs [19]. The multivariate analysis identified the presence of SRCs, micropapillary-predominant histology, and hepatoid tumour cells as the most predictive independent factors of ALK rearrangements. Further molecular analysis showed no concurrent activating mutations in EGFR or KRAS detected in all the ALK positive cases, similar to our case.
Prognosis remains poor for patients with pulmonary adenocarcinoma with SRC morphology. This was clearly evidenced by previous studies. Wu SG et al., analysed a total of 738 cases and found that most patients had a poor differentiation status, had distant metastasis at the initial diagnosis, with the five-year Cancer-Specific Survival (CSS) rate being only 11% and a median CSS of six months [20]. Tsuta K et al., analysed the 5-year survival rates of patients non SRCC, with L-SRCC, or with H-SRCC which were 52.7%, 50%, and 28.4%, respectively, which indicates the aggressiveness of the tumour being directly proportional to the percentage of the SRC component [14]. This finding is in correlation with the study by Chen C et al., as well, who found that lung adenocarcinomas with SRC component has worse Recurrence-Free Survival (RFS) (p<0.001) and Overall Survival (OS) (p<0.001) [14].
Conclusion(s)
This case report highlights the rarity of the incidence of lung adenocarcinoma with SRC morphology. A careful analysis of the specimen from endobronchial fluid is crucial to suspect the presence of SRCs, and further histopathological diagnosis of this rare entity is essential. However, a metastatic SRC carcinoma from other sites must be considered, and hence a multidisciplinary approach is essential, with proper correlation with radiological investigations like PET/CT, which is imperative to better plan patient management. The immunohistochemical features and association of the ALK gene rearrangement is also emphasised. Given the aggressiveness of this tumour and poor prognosis, frequent lymph nodal metastasis, and its association with non smokers, a thorough history and examination is essential to alleviate the late diagnosis which could further curtail the survival in these patients.
[1]. GLOBOCAN UNew global cancer dataUICC 2020 27:2022 [Google Scholar]
[2]. Deshpand R, Chandra M, Rauthan A, Evolving trends in lung cancer: Epidemiology, diagnosis, and managementIndian J Cancer 2022 59(Suppl 1):S90-105.10.4103/ijc.IJC_52_2135343194 [Google Scholar] [CrossRef] [PubMed]
[3]. Succony L, Rassl DM, Barker AP, McCaughan FM, Rintoul RC, Adenocarcinoma spectrum lesions of the lung: Detection, pathology and treatment strategiesCancer Treat Rev 2021 99:10223710.1016/j.ctrv.2021.10223734182217 [Google Scholar] [CrossRef] [PubMed]
[4]. Kish JK, Ro JY, Ayala AG, McMurtrey MJ, Primary mucinous adenocarcinoma of the lung with signet-ring cells: A histochemical comparison with signet-ring cell carcinomas of other sitesHum Pathol 1989 20(11):1097-102.10.1016/0046-8177(89)90229-32478443 [Google Scholar] [CrossRef] [PubMed]
[5]. Travis WD, Brambilla E, Müller-Hermelink HK, Harris CC, World Health Organization Classification of TumoursPathology and Genetics of Tumours of the Lung, Pleura, Thymus and Heart 2004 103rd EditionLyonIARC Press:344 [Google Scholar]
[6]. Nicholson AG, Tsao MS, Beasley MB, Borczuk AC, Brambilla E, Cooper WA, The 2021 WHO Classification of lung tumors: Impact of advances since 2015J Thorac Oncol 2022 17(3):362-87.10.1016/j.jtho.2021.11.00334808341 [Google Scholar] [CrossRef] [PubMed]
[7]. Boland JM, Wampfler JA, Jang JS, Wang X, Erickson-Johnson MR, Oliveira AM, Pulmonary adenocarcinoma with signet ring cell features: A comprehensive study from 3 distinct patient cohortsAm J Surg Pathol 2014 38(12):1681-88.10.1097/PAS.000000000000028025007143 [Google Scholar] [CrossRef] [PubMed]
[8]. Tan Y, Lu X, Li Y, Liao D, Xie W, Song J, ALK-positive pulmonary adenocarcinoma with signet ring features (PASRF) and polygonal cell morphology simultaneously co-expressing TTF-1/p63/P40: A case reportTransl Cancer Res TCR 2021 10(8):3864-69.10.21037/tcr-21-33535116685PMC8797292 [Google Scholar] [CrossRef] [PubMed]
[9]. Merchant SH, Amin MB, Tamboli P, Ro J, Ordóñez NG, Ayala AG, Primary signet-ring cell carcinoma of lung: Immunohistochemical study and comparison with non-pulmonary signet-ring cell carcinomasAm J Surg Pathol 2001 25(12):1515-19.10.1097/00000478-200112000-0000711717541 [Google Scholar] [CrossRef] [PubMed]
[10]. Al-Taee A, Almukhtar R, Lai J, Jallad B, Metastatic signet ring cell carcinoma of unknown primary origin: A case report and review of the literatureAnn Transl Med 2016 4(15):28310.21037/atm.2016.07.2427570777PMC4980384 [Google Scholar] [CrossRef] [PubMed]
[11]. Gregoire C, Muller G, Machiels JP, Goeminne JC, Metastatic signet-ring cell carcinoma of unknown primary originActa Clinica Belgica 2014 69(2):135-38.10.1179/0001551213Z.000000000224724758 [Google Scholar] [CrossRef] [PubMed]
[12]. Organisation mondiale de la santé, Centre international de recherche sur le cancer, editorsThoracic tumours 2021 5th edLyonInternational agency for research on cancer(World health organization classification of tumours) [Google Scholar]
[13]. Vallonthaiel AG, Jain D, Madan K, Arava S, Pulmonary adenocarcinoma with signet ring features: Detailed cytomorphologic analysisDiagn Cytopathol 2016 44(7):607-11.10.1002/dc.2349227095297 [Google Scholar] [CrossRef] [PubMed]
[14]. Tsuta K, Ishii G, Yoh K, Nitadori J, Hasebe T, Nishiwaki Y, Primary lung carcinoma with signet-ring cell carcinoma components: Clinicopathological analysis of 39 casesAm J Surg Pathol 2004 28(7):868-74.10.1097/00000478-200407000-0000415223955 [Google Scholar] [CrossRef] [PubMed]
[15]. Chen C, Wang L, Gu C, Wang Y, Pan X, Fu S, Survival analyses and immunohistochemical study of primary signet ring cell carcinoma of the lung adenocarcinomaTransl Cancer Res TCR 2020 9(2):620-28.10.21037/tcr.2019.11.5435117407PMC8797340 [Google Scholar] [CrossRef] [PubMed]
[16]. Muscarella LA, Trombetta D, Fabrizio FP, Scarpa A, Fazio VM, Maiello E, ALK and NRG1 fusions coexist in a patient with signet ring cell lung adenocarcinomaJ Thorac Oncol 2017 12(10):e161-e163.10.1016/j.jtho.2017.05.01428939148 [Google Scholar] [CrossRef] [PubMed]
[17]. Possidente L, Landriscina M, Patitucci G, Borgia L, Lalinga V, Vita G, ALK rearrangement in specific subtypes of lung adenocarcinoma: Immunophenotypic and morphological featuresMed Oncol 2017 34(5):7610.1007/s12032-017-0936-z28364271 [Google Scholar] [CrossRef] [PubMed]
[18]. Ha SY, Ahn J, Roh MS, Han J, Lee JJ, Lee B, Cytologic features of ALK-positive pulmonary adenocarcinomaKorean J Pathol 2013 47(3):25210.4132/KoreanJPathol.2013.47.3.25223837018PMC3701821 [Google Scholar] [CrossRef] [PubMed]
[19]. Nishino M, Klepeis VE, Yeap BY, Bergethon K, Morales-Oyarvide V, Dias-Santagata D, Histologic and cytomorphologic features of ALK-rearranged lung adenocarcinomasMod Pathol 2012 25(11):1462-72.10.1038/modpathol.2012.10922743652 [Google Scholar] [CrossRef] [PubMed]
[20]. Wu SG, Chen XT, Zhang WW, Sun JY, Li FY, He ZY, Survival in signet ring cell carcinoma varies based on primary tumor location: A surveillance, epidemiology, and end Results database analysisExpert Rev Gastroenterol Hepatol 2018 12(2):209-14.10.1080/17474124.2018.141629129227748 [Google Scholar] [CrossRef] [PubMed]