The SCB plexus block for upper limb surgeries has emerged as a safe technique, with a rapid and reliable onset compared to general anaesthesia; it is being considered a spinal technique for the upper limb. Various techniques have been documented in the literature for SCB, but the use of US for the administration of SCB improves accuracy and success rates (95%). It has emerged as a safer and more effective technique [1-5]. Ropivacaine provides a good amount of pain relief with less motor blockade, as it has a greater degree of motor-to-sensory differentiation, which helps in early postoperative mobilisation. Additionally, it has less cardiotoxic effect than bupivacaine, making it more suitable for SCB [1,2].
Various adjuvants have been tried in SCB, as they extend the period of analgesia and reduce the local anaesthetic dose requirement, thereby minimising systemic adverse effects. Dexmedetomidine is one of these adjuvants, with a highly selective α-2 agonistic activity (α-2 to α-1 activity 1620:1) compared to Clonidine (α-2 to α-1 activity 220:1) and it does not cause respiratory depression [1,2,6]. Dexmedetomidine binds and inhibits the release of nor adrenaline from presynaptic α-2 receptors in the sympathetic nervous system and non adrenergic receptors in the central nervous system [1].
Dexmedetomidine has a profound anxiolytic and sedative effect by acting on the locus coeruleus, with no respiratory depression. It reduces sympathetic tone and attenuates the neuroendocrine and haemodynamic response to anaesthesia and surgery. It decreases intraoperative anaesthetic requirements by improving sedation and analgesia, leading to better patient satisfaction. Moreover, it reduces postoperative analgesic requirements in those painful procedures. When used as an adjuvant in regional anaesthesia along with a local anaesthetic at a dose of 1 μg/kg, dexmedetomidine improves the quality of intraoperative anaesthesia and postoperative analgesia, as well as enhances cardiovascular stability without any adverse effects [7-10].
A lower dose of dexmedetomidine does not provide adequate analgesia, while higher doses can cause unusual bradycardia and hypotension. The optimal dose of dexmedetomidine as an adjuvant in regional nerve blocks is yet to be determined [11,12]. The literature on dexmedetomidine dosing as an adjuvant in SCB is limited; to our knowledge, there have only been five studies conducted using dexmedetomidine in SCB with ropivacaine. Out of these, only one study has compared a dose of 25 μg of dexmedetomidine in addition to ropivacaine in SCB [1,13-15]. This study focused on examining the effect of adding dexmedetomidine as an adjuvant to ropivacaine in SCB on intraoperative and postoperative analgesia during upper limb surgeries. The primary objective of the study was to evaluate the onset and duration of SCB plexus block, while the secondary objective was to assess haemodynamic stability and drug-related side-effects.
Materials and Methods
This was a randomised controlled study conducted Osmania Medical College and General Hospital, Hyderabad, Telangana, India over a period of one year during June 2019 to May 2020. Institutional Ethical Committee (IEC) clearance was obtained (ECR/300/Inst/AP/2013/RR-19), informed and written consent was obtained from the participants.
Inclusion criteria: Subjects aged between 18 and 60 years, ASA I and II, undergoing upper limb surgeries, were included in the study.
Exclusion criteria: Subjects with ASA III and IV classifications, bleeding disorders, nerve injuries, neuropathy and pneumothorax were excluded from the study.
Sample size calculation: The sample size was calculated based on a previous study by Dash LK et al., considering the mean difference in the time of onset of motor blockade to be 4.38 minutes, with an anticipated standard deviation of 5.83 minutes, at a significance level of 5% and a power of 80% [13]. Based on this data, 28 patients were required in each group, assuming a screen failure rate of 7%. Accordingly, 30 patients were recruited and randomly allocated into two groups using the “slips in box technique” (group R - Control group and group RD - Test group) [Table/Fig-1].
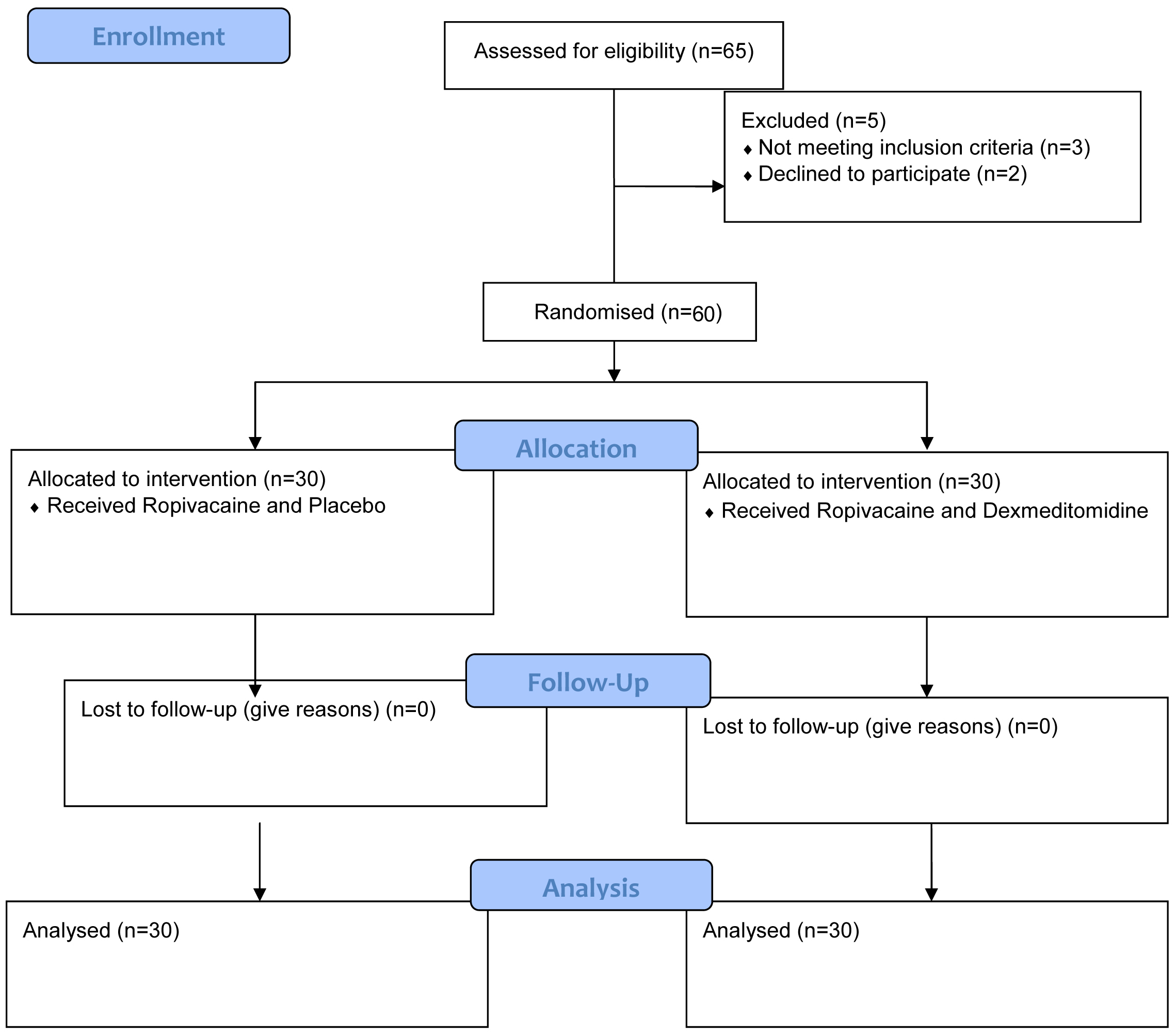
Study Procedure
All subjects underwent a preanaesthesia evaluation and the orders were followed. The subjects were positioned supine with their heads turned to the contralateral side after securing intravenous access in the non surgical hand. The SCB plexus block was performed under all aseptic precautions with the assistance of US. Group R subjects received 30 mL of 0.5% ropivacaine and 1 mL of saline, while group RD subjects received 30 mL of 0.5% ropivacaine and 25 μg of dexmedetomidine mixed with saline to make a total volume of 1 mL [1].
Sensory block was assessed using a pinprick three-point scale: {0 - Normal sensation, 1 - Loss of sensation to pinprick (analgesia), 2 - Loss of sensation to touch (anaesthesia)}. Motor blockade was assessed using the Modified Bromage Scale: {Grade 0 - No block, total arm and forearm flexion; Grade I - Partial block, total forearm and partial arm flexion; Grade II - Almost complete block, inability to flex the arm and decreased ability to flex the forearm; Grade III - Total block, inability to flex both arm and forearm} after the injection of the drug, assessed every two minutes until 30 minutes.
The onset of sensory block (the interval between the administration of the drug and complete sensory block), the onset of motor block (the interval between the administration of the drug and complete motor block), the duration of sensory block (the interval between the onset of sensory block and the first rescue analgesia) and the duration of motor block (the interval between the onset of motor block and complete recovery of power) were assessed. Haemodynamic monitoring was conducted every five minutes for the first hour and every 15 minutes until the end of the procedure. Adverse events, such as nausea, vomiting, sedation and respiratory depression, if any, were noted.
Statistical Analysis
Data was collected and tabulated into an Excel sheet. The data was expressed as means and percentages. Statistical analysis was conducted using Statistical Package for the Social Sciences {SPSS version 21.0 (14 days free trial)}, specifically through an unpaired t-test and the results are documented.
Results
Both study groups were comparable in demographic characteristics such as age, weight, height and ASA grade [Table/Fig-2].
Demographic profile of the patients.
| Demographic profile | Group R (n=30) | Group RD (n=30) | p-value |
|---|
| Mean±SD | Mean±SD |
|---|
| Age (years) | 33.10±8.73 | 30.53±7.77 | 0.234 |
| Weight (kg) | 69.16±4.35 | 68.14±4.2 | 0.9 |
| Height (cm) | 158±4.2 | 159±3.81 | 0.78 |
| Gender ratio (M:F) | 25 : 5 | 23 : 7 | 0.81 |
| ASA Grade (I/II) | 21/09 | 22/08 | 0.85 |
The onset of sensory block was faster in group RD compared to group R. Similarly, the onset of motor block was also earlier in group RD when compared to group R. The mean duration of sensory block was significantly longer in group RD than in group R. Likewise, the mean duration of motor block was also significantly longer in group RD compared to group R [Table/Fig-3].
Onset time and duration of sensory, motor block and duration of surgery.
| Variables | Group R (n=30) | Group RD (n=30) | p-value |
|---|
| Mean±SD | Mean±SD |
|---|
| Onset of sensory block (in mins) | 7.87±1.98 | 4.78±1.68 | <0.001 |
| Onset of motor block (in mins) | 12.3±2.95 | 8.4±2.34 | <0.001 |
| Duration of sensory block (in mins) | 485±81.31 | 807.5±165.51 | <0.001 |
| Duration of motor block (in mins) | 465±72.62 | 685±62.74 | <0.001 |
| Duration of surgery (in mins) | 162±3.74 | 161±3.21 | 0.91 |
The haemodynamic parameters, such as heart rate, systolic blood pressure and diastolic blood pressure, were similar in both groups intraoperatively and good haemodynamic stability was observed in both groups. There was significant difference in systolic and diastolic blood pressures between the groups. Additionally, there were no incidences of bradycardia, hypotension, or other side-effects in either group [Table/Fig-4].
Observation of haemodynamic variables among both the groups.
| Variable | Heart rate (bpm) | Systolic BP (mmHg) | Diastolic BP (mmHg) |
|---|
| Time | Group R | Group RD | p-value | Group R | Group RD | p-value | Group R | Group RD | p-value |
|---|
| Pre OP | 92.2±3.2 | 91.4±4.5 | 0.401 | 131.13±7.19 | 128.9±11.59 | 0.373 | 84±7.89 | 81.27±7.8 | 0.182 |
| 15 mins | 86.3±7.3 | 76.4±10.96 | <0.001 | 130.83±6.85 | 126.13±15.52 | 0.135 | 83.50±5.19 | 75.27±8.13 | <0.001 |
| 30 mins | 82±8.76 | 70.4±10 | <0.001 | 128.47±6.82 | 119.07±12.02 | <0.001 | 81.1±6.75 | 72.1±9.64 | <0.001 |
| 60 mins | 83.2±9.16 | 69.87±10.2 | <0.001 | 127.77±7.61 | 117.7±13.32 | <0.001 | 80.27±7.05 | 71.77±8.61 | <0.001 |
| 120 mins | 82.7±7.4 | 71.5±11.7 | <0.001 | 128.9±6.43 | 116.87±12.32 | <0.001 | 79.97±5.63 | 71.5±7.31 | <0.001 |
Discussion
Dexmedetomidine is being used for intravenous sedation, analgesia, regional anaesthesia and even in intensive care units for sedation. Its use in spinal and epidural anaesthesia as an adjuvant has increased in the recent past. Literature on its usage in SCB as an adjuvant is limited. Present study investigated the effect of adding dexmedetomidine to ropivacaine on the quality of anaesthesia [1,7,16]. Ropivacaine is comparable to bupivacaine in all aspects, except for its decreased motor blockade, which helps in early, pain-free mobilisation of the limb. The duration of analgesia is shorter with the plain usage of local anesthetic in SCB compared to the utilisation of adjuvants. Dexmedetomidine is an excellent adjuvant to local anaesthetics for prolonging the duration of analgesia in SCB [17,18].
In present study, demographic data regarding age, height, weight, gender and ASA physical status were comparable and the differences between the parameters among both groups were statistically not significant, which was similar to findings in other studies as well [1,13]. In present study, the ropivacaine with dexmedetomidine group (group RD) showed a faster onset of sensory block compared to the plain Ropivacaine group (group R) (4.78±1.68 mins vs. 7.87±1.98 mins, p-value <0.001). Similarly, the onset of motor blockade was earlier in group RD than in group R (8.4±2.34 mins vs. 12.3±2.95 mins, p-value <0.001). Similar findings were observed when using Dexmedetomidine as an adjuvant to Bupivacaine in SCB by Agarwal S et al., [19]. Jun Z et al., also recorded similar findings in their study by using dexmedetomidine as an adjuvant to ropivacaine. Additionally, they concluded that dexmedetomidine reduced upper limb ischaemia-reperfusion injury caused by the tourniquet [20]. Pandya N et al., also observed a faster onset, longer duration of blockade and longer duration of analgesia [15].
Present study analysed the effect of 25 μg of dexmedetomidine as an adjuvant to 30 mL of ropivacaine, as this dose of dexmedetomidine has been shown to reduce the chances of bradycardia and hypotension compared to higher doses, while still providing similar effects on sensory and motor block, analgesia and enhanced quality of the block. This dosing of ropivacaine has been studied in various other studies in SCB [21-24]. In present study, the mean duration of motor blockade was significantly longer in group RD than in group R (685±62.74 mins vs. 465±72.62 mins, p-value <0.001). Similarly, the duration of analgesia was longer in group RD than in group R (807.5±165.51 mins vs. 485±81.31 mins, p-value <0.001). Similar findings were documented by Sudani C et al., and Pandya N et al., who concluded that the utilisation of dexmedetomidine as an adjuvant to ropivacaine in SCB prolongs the duration of motor blockade and analgesia [1,15].
Limitation(s)
As this study was conducted at a single centre, the findings may not be generalised. Large multricentric studies should be conducted in future for more generalised results.
Conclusion(s)
Dexmedetomidine, administered as an adjuvant at a dose of 25 μg in combination with ropivacaine during an ultrasound-guided SCB, demonstrates a faster onset of sensory and motor block. It also prolongs the duration of sensory blockade, motor blockade and analgesia, resulting in decreased postoperative analgesic requirements and providing a high-quality block for patients. Additionally, it offers excellent haemodynamic stability with minimal complications, positioning dexmedetomidine as one of the best adjuvants to ropivacaine for SCB.