Introduction
With Type 2 Diabetes Mellitus (T2DM) affecting over 74 million individuals in India alone, achieving glycaemic control remains a persistent challenge, especially in patients inadequately managed with Oral Hypoglycaemic Agents (OHAs). The present study explored the potential of imeglimin, a novel antidiabetic drug targeting mitochondrial function, to bridge this gap. By addressing insulin resistance and β-cell dysfunction, imeglimin emerges as a dual-action therapy tailored for patients who are hesitant to initiate insulin.
Aim
To assess the short-term effectiveness and safety of imeglimin in T2DM patients who are inadequately controlled on two or three OHAs and who are hesitant to initiate insulin therapy.
Materials and Methods
The present prospective, single-arm, single-centre interventional study was conducted in the Department of Endocrinology, Harmony Dr. Sachin’s 360 Degree Diabetes Care Centre, Bhopal, Madhya Pradesh, India, from January 2023 to June 2023. A total of 75 patients meeting the inclusion criteria were enrolled. Baseline and 12-week post-treatment data were systematically recorded, including demographic variables, anthropometric measurements {height, weight, and Body Mass Index (BMI)}, and glycaemic parameters {Fasting Plasma Glucose (FPG), Postprandial Glucose (PPG), and Glycated Haemoglobin (HbA1c)}. Statistical analysis was conducted using one-way ANOVA to evaluate treatment effects.
Results
The study population comprised 75 participants with a mean±Standard Deviation (SD) age of 57±10 years and a duration of diabetes diagnosis ranging from six months to 25 years. Males represented a predominant 54.6% of the study cohort. Following 12 weeks of imeglimin treatment, HbA1c levels demonstrated a mean reduction of 1.1% (p-value=0.004) compared to baseline values. Furthermore, FPG levels decreased by a mean of 49.3 mg/dL (p-value=0.013), while PPG exhibited a mean reduction of 79.4 mg/dL (p-value=0.020). The most frequently reported treatment-emergent adverse events were gastrointestinal in nature.
Conclusion
Imeglimin demonstrated short-term effectiveness in enhancing glycaemic control as an adjunct therapy to existing OHAs in T2DM patients who are hesitant to initiate insulin. The treatment was well tolerated, with gastrointestinal issues being the primary adverse effect. Further extensive studies with larger sample sizes are warranted to validate these findings and ascertain the long-term effects of imeglimin in T2DM management.
Introduction
The T2DM is a chronic, progressive metabolic disorder primarily characterised by insulin resistance and impaired pancreatic β-cell function, leading to persistent hyperglycaemia [1]. T2DM represents a significant global health burden, affecting an estimated 463 million adults worldwide in 2019, with increasing obesity and aging populations driving its prevalence. In India, for example, the diabetic population reached 74.9 million in 2021, a figure projected to rise alarmingly to 124.9 million by 2045 [2]. Chronic hyperglycaemia associated with T2DM often leads to severe complications such as retinopathy, nephropathy, neuropathy, and an increased risk of cardiovascular disease [1].
Management of T2DM involves both lifestyle modifications and pharmacological interventions aimed at improving glycaemic control [3] and often requires a multifaceted approach due to the complex pathophysiology of the disease. Despite the availability of various OHAs for managing T2DM, achieving and maintaining optimal glycaemic control remains a significant challenge. Many patients do not reach target HbA1c levels with existing monotherapies or combination treatments, highlighting a critical gap in effective diabetes management strategies [4]. In this context, combination therapies have emerged as an effective strategy to target multiple mechanisms contributing to hyperglycaemia [5].
Imeglimin, a novel oral agent, has demonstrated significant efficacy in combination with other OHAs, addressing key pathophysiological defects in T2DM [6]. Its dual mechanism of action, enhancing insulin secretion in response to glucose and improving insulin sensitivity in peripheral tissues, makes it particularly beneficial when used alongside other agents such as metformin, Sulfonylureas (SU), and Dipeptidyl Peptidase-4 (DPP-4) inhibitors [7]. Clinical trials have consistently shown that imeglimin enhances the glycaemic control provided by these drugs, reducing HbA1c levels more effectively than monotherapy [8].
Furthermore, imeglimin’s ability to reduce hepatic glucose production and promote mitochondrial function distinguishes it from other therapies, making it a versatile option in combination regimens. Studies such as the Trial for Imeglimin Efficacy and Safety 2 (TIMES 2) and TIMES 3 trials have demonstrated significant improvements in glycaemic outcomes when imeglimin is used as an add-on therapy in patients who previously had inadequate control on existing medications [9,10]. These results highlight imeglimin’s potential to address an important unmet need in diabetes management, especially for patients who have difficulty reaching glycaemic goals with standard treatments.
A major challenge in the treatment of T2DM is the progressive β-cell dysfunction and failure, a key pathogenic feature of the disease [11]. Over time, the β-cells’ ability to compensate for insulin resistance diminishes, leading to worsening glycaemic control [12]. As a result, a substantial proportion of patients more than 60% fail to achieve recommended glycaemic targets, with HbA1c levels remaining above 7%, even with treatment [13]. The emergence of novel therapeutic options, including new OHAs, has expanded the treatment landscape for T2DM [14]. OHAs target various pathophysiological defects contributing to hyperglycaemia, employing mechanisms such as stimulating insulin secretion, enhancing insulin sensitivity, delaying glucose absorption, and promoting renal glucose excretion [15,16].
The present study was aimed to evaluate the short-term efficacy and safety of imeglimin as an adjunctive therapy to existing OHAs in improving glycaemic control among patients with inadequately controlled T2DM in Central India. By exploring the impact of imeglimin on β-cell function and insulin sensitivity, the present research contributes to the growing body of evidence supporting mitochondrial-targeted therapies in T2DM management.
Materials and Methods
The present prospective, single-arm, single-centre interventional study was conducted in the Department of Endocrinology, Harmony Dr. Sachin’s 360 Degree Diabetes Care Centre, Bhopal, Madhya Pradesh, India, from January 2023 to June 2023. The study included 75 patients with confirmed T2DM aged between 33 years and 83 years, who were receiving routine care at the facility. Ethical approval was obtained from the Institutional Ethics Committee of Sri Aurobindo Institute of Medical Sciences (IEC No. SAIMS/IEC/08/23), and the study adhered to the International Council for Harmonisation Good Clinical Practice (ICH-GCP) guidelines. Written informed consent was obtained from all participants prior to their enrollment in the study.
Inclusion criteria: Patients who had previously received treatment with two or three distinct OHAs yet exhibited suboptimal glycaemic regulation, as indicated by elevated levels of glycated haemoglobin (HbA1c), FPG and PPG exceeding the targeted therapeutic ranges were included in the study. Individuals reluctant to initiate insulin therapy were also included in the study.
Exclusion criteria: All the patients with a known allergy or hypersensitivity to imeglimin, those who were unable or unwilling to provide informed consent, and pregnant females with gestational diabetes mellitus were excluded from the study. No patients were excluded based on these criteria during the study period.
Study Procedure
All the included patients were given oral imeglimin at a dose of 2000 mg per day [17] as a third or fourth-line therapeutic intervention for glycaemic control over a duration of three months. Patients with suboptimal glycaemic regulation, even after imeglimin administration during the study period, were subsequently prescribed insulin therapy and consequently excluded from the study cohort. Patients’ demographic information, including age, gender and occupation, was recorded, along with the anthropometric measurements like height, weight and BMI at baseline. The glycaemic parameters, including FPG, PPG and HbA1c, were collected and analysed. The presence of diabetic complications, including retinopathy, neuropathy and nephropathy, was assessed among the study participants. The classification of neuropathy into normal, mild, moderate and severe was based on clinical presentation, physical examination, and nerve conduction study findings. Similarly, nephropathy was categorised using the Kidney Disease Improving Global Outcomes (KDIGO) guidelines, considering the Albumin-to-Creatinine Ratio (ACR) and estimated Glomerular Filtration Rate (eGFR) [18].
Statistical Analysis
Quantitative variables were presented as means and Standard Deviation (SD), while qualitative variables were expressed as frequency and proportions. A paired t-test was performed to find the difference in means, with a significance level of p-value <0.05 considered indicative of statistical significance. The statistical analysis was done using Statistical Package for the Social Sciences (SPSS) software version 26.0.
Results
A total of seventy-five patients were included in this single-centre study. Demographic characteristics such as age, gender, family history and occupation were presented in [Table/Fig-1]. The duration of diabetes diagnosis among the participants ranged from six months to 25 years. Out of the 75 patients, more than half (53.4%) were diagnosed with neuropathy [Table/Fig-2].
Demographic profile of the patients (N=75).
| Characteristics | Observed value |
|---|
| Age (years) (mean±SD) | 57±10 |
| Height (cm) (mean±SD) | 161±9.20 |
| Weight (kg) (mean±SD) | 70±12.27 |
| BMI (kg/m2) (mean±SD) | 27±4.21 |
| Gender, n (%) |
| Male | 41 (54.6) |
| Female | 34 (45.3) |
| Family history of diabetes, n (%) |
| Yes | 14 (18.6) |
| No | 61 (81.3) |
| Profession, n (%) |
| Homemaker/unemployed | 28 (37.3) |
| Working | 34 (45. 3) |
| Retired (M) | 13 (17.3) |
Observed values of diabetic complications in patients under study.
| Parameters | Category | Observed values n (%) |
|---|
| Neuropathy | Normal | 35 (46.6) |
| Mild loss of Vibratory perception | 20 (26.6) |
| Moderate loss of Vibratory perception | 3 (4.0) |
| Severe loss of Vibratory perception | 17 (22.6) |
| Albuminuria (Nephropathy) | Normal (<30 mg/g) | 23 (30.6) |
| Moderately increased (30-299 mg/g) | 15 (20.0) |
| Severely increased (≥300 mg/g) | 37 (49.3) |
The intervention demonstrated significant reductions in HbA1c from 9.1±1.8% to 8.0±1.4% (p-value=0.004), FPG from 171.3±43.8 to 122.0±26.2 mg/dL (p-value=0.013), and PPG from 250.4±64.7 to 171.0±27.2 mg/dL (p-value=0.020), respectively. These improvements across key glycaemic parameters suggested enhanced glycaemic control and potential benefits in managing T2DM [Table/Fig-3].
Effects of imeglimin on glycaemic parameters.
| Parameter | Baseline (mean±SD) | After 12 weeks of imeglimin administration (mean±SD) | p-value |
|---|
| HbA1c (%) | 9.1±1.8 | 8.0±1.4 | 0.004 |
| FPG (mg/dL) | 171.3±43.8 | 122.0±26.2 | 0.013 |
| PPG (mg/dL) | 250.4±64.7 | 171.0±27.2 | 0.020 |
Statistical analysis for the glycaemic parameters presented in this table was conducted using One-way ANOVA
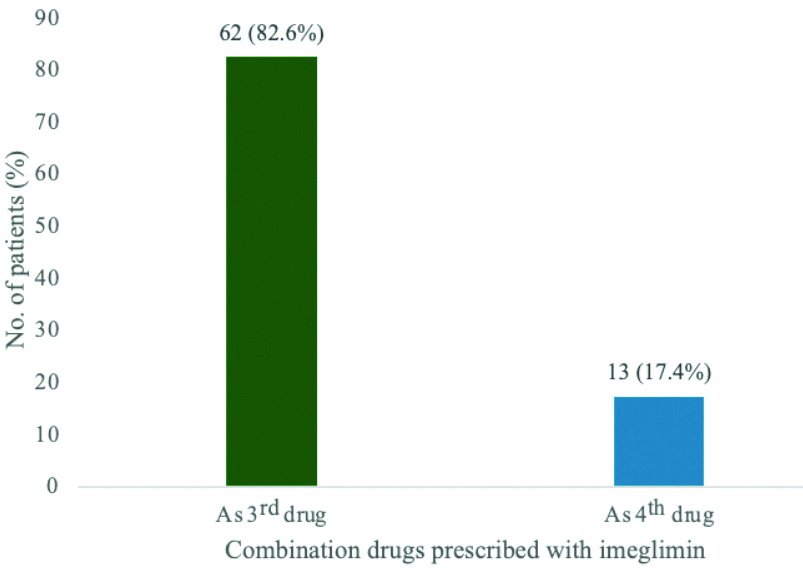
Before initiating imeglimin therapy, 62 (82.6%) patients were prescribed a combination regimen consisting of SUs with biguanides, Dipeptidyl Peptidase 4 inhibitors (DPP4-I) with Sodium Glucose cotransporter 2 inhibitors (SGLT2-I), while the remaining 13 (17.4%) patients were on a combination of SUs with biguanides, DPP4-I with SGLT2-I and Alpha-Glucosidase Inhibitors (AGI) [Table/Fig-4].
Patients taken imeglimin in combination with other OHAs; As 3rd drug=(SU+biguanides)+(Dipeptidyl peptidase 4 inhibitors+sodium glucose cotransporter 2.
Among all 75 patients, gastrointestinal adverse effects were reported in 21 (28.14%) of the patients. Nausea was the most frequently observed adverse effect, reported by nine patients, followed by abdominal pain in seven patients [Table/Fig-5].
Adverse effects in the patients.
| Adverse effects | No. of patients (n) |
|---|
| Nausea | 9 |
| Abdominal pain | 7 |
| Constipation | 2 |
| Diarrhoea and vomiting | 3 |
Discussion
Imeglimin’s unique mechanism of action offers therapeutic advantages in both monotherapy and combination therapy settings. When administered as monotherapy, imeglimin has shown moderate glycaemic control. In the TIMES 1 trial, imeglimin monotherapy led to a reduction in HbA1c levels by approximately 0.6% over 24 weeks in patients with baseline HbA1c levels of 7.9-8.5% [19]. This suggests that while imeglimin is effective as monotherapy, its efficacy may be limited in patients with advanced disease who require additional therapeutic interventions.
In contrast, when imeglimin is combined with other OHAs, its efficacy in lowering HbA1c levels is significantly enhanced. In the TIMES 2 study, imeglimin, as an add-on therapy to existing oral antidiabetics, demonstrated an HbA1c reduction of up to 1.0%, depending on the combination, with the most significant reductions observed when combined with metformin and DPP-4 inhibitors [10].
The findings demonstrated a significant reduction in HbA1c levels from 9.1% to 8.0% when imeglimin was used as an adjunct to other OHAs. This reduction is consistent with prior research, including a real-world observational study {the Glycemic Control Evaluation in Specific Population/Intervention (GLYCEM-IN) Trial}, which demonstrated significant reductions in glycaemic parameters when imeglimin was used as a third-line agent for T2DM patients inadequately controlled on other therapies. A mean reduction in FPG by 104.7 mg/dL, PPG by 117.7 mg/dL, and HbA1c levels by 0.45% (all p-value <0.0001) was observed, indicating the potential of imeglimin as a valuable addition to combination therapy in the management of T2DM.
The findings of the present study are consistent with the results reported by Fouqueray P et al., who demonstrated a significant reduction in HbA1c of 0.72% when imeglimin was added to sitagliptin therapy compared to sitagliptin monotherapy. This incremental reduction highlights the potential of imeglimin to act as a robust add-on therapy, particularly in patients with type 2 diabetes inadequately controlled with DPP-4 inhibitors alone [20]. Similarly, Dubourg J et al., reported varying degrees of HbA1c reduction when imeglimin was co-administered with different classes of OHAs, with a mean reduction ranging from 0.12% with GLP-1 receptor agonists to 0.92% with DPP-4 inhibitors [10].
In addition to glycaemic control, the safety profile of imeglimin remains a key consideration, particularly its low risk of hypoglycaemia. Previous studies, including TIMES 1 and TIMES three trials, demonstrated that imeglimin has a low risk of inducing hypoglycaemia when used either as monotherapy or in combination with non-insulin secretagogues, such as metformin or DPP-4 inhibitors. This is due to its glucose-dependent insulinotropic effect, which ensures that insulin secretion is stimulated only in the presence of elevated glucose levels [9,19]. However, in the present study, one hypoglycaemic event (1.33%) was observed, which may be attributed to the concomitant use of SU, which can stimulate insulin secretion regardless of glucose levels.
Consistent with previous studies, the most common adverse events associated with imeglimin in the present study were gastrointestinal in nature, including nausea, abdominal pain and diarrhoea. These findings align with studies by Bouchoucha M et al., and Oda T et al., where similar patterns of gastrointestinal side effects were observed, particularly when imeglimin was combined with metformin. While these adverse events were mostly mild and temporary, they emphasise the need for careful monitoring of patient tolerance, especially when combined with metformin, which is also associated with gastrointestinal side effects [21,22].
No real-world studies have yet assessed imeglimin’s impact on glycaemic control with other OHA in T2D. This first-in-class oral agent acts through modulation of mitochondrial bioenergetics, addressing mitochondrial dysfunction, a known contributor to β-cells dysfunction and the progression of T2D. Similarly, the MEGMI study showed a reduction in HbA1c of at least 0.87% when imeglimin was added to DPP-4 inhibitors and low-dose metformin, highlighting its role as an effective add-on therapy [17].
Published meta-analyses and clinical trials provide additional context to the findings of this study. Dubourg J et al., reported enhanced glycaemic outcomes when imeglimin was combined with DPP-4 inhibitors or metformin, with HbA1c reductions of up to 0.92% and 1.0%, respectively [23]. Similarly, Fouqueray P et al., emphasised the dual mechanism of imeglimin, which enhances β-cell function and insulin sensitivity, thereby offering a therapeutic advantage in managing complex pathophysiological aspects of T2D [24]. The current study corroborates these findings, as it also highlights significant improvements in glycaemic parameters without an increased risk of hypoglycaemia.
Limitation(s)
However, the present study’s retrospective design and relatively small sample size limit the ability to draw definitive conclusions regarding the long-term efficacy and safety of imeglimin, particularly in combination with insulin secretagogues.
Conclusion(s)
The combination of imeglimin with oral antidiabetic medications was well tolerated by patients with T2DM and led to clinically significant and sustained reductions in hyperglycaemic blood parameters. The present study highlights the efficacy and safety of imeglimin as an adjunctive therapy in patients with T2D who are inadequately controlled on two or three OHAs. These findings highlight the potential of imeglimin to address insulin resistance and β-cell dysfunction, providing a promising therapeutic option for patients who are reluctant to initiate insulin therapy.
Statistical analysis for the glycaemic parameters presented in this table was conducted using One-way ANOVA
[1]. Pirags V, Lebovitz H, Fouqueray P, Imeglimin, a novel glimin oral antidiabetic, exhibits a good efficacy and safety profile in type 2 diabetic patientsDiabetes Obes Metab 2012 14(9):852-58.10.1111/j.1463-1326.2012.01611.x22519919 [Google Scholar] [CrossRef] [PubMed]
[2]. Borse SP, Chhipa AS, Sharma V, Singh DP, Nivsarkar M, Management of type 2 diabetes: Current strategies, unfocussed aspects, challenges, and alternativesMed Princ Pract 2021 30(2):109-21.10.1159/00051100232818934PMC8114074 [Google Scholar] [CrossRef] [PubMed]
[3]. Patel R, Sina RE, Keyes D, Lifestyle Modification for Diabetes and Heart Disease PreventionIn: StatPearls [Internet] 2024 Treasure Island (FL)StatPearls Publishing[cited 2024 Dec 26]. Available from: http://www.ncbi.nlm.nih.gov/books/NBK585052/ [Google Scholar]
[4]. Moon MK, Hur KY, Ko SH, Park SO, Lee BW, Kim JH, Combination therapy of oral hypoglycaemic agents in patients with type 2 diabetes mellitusKorean J Intern Med 2017 32(6):974-83.10.3904/kjim.2017.35429096431 [Google Scholar] [CrossRef] [PubMed]
[5]. Chaudhury A, Duvoor C, Reddy Dendi VS, Kraleti S, Chada A, Ravilla R, Clinical review of antidiabetic drugs: Implications for type 2 diabetes mellitus managementFront Endocrinol (Lausanne) 2017 8:610.3389/fendo.2017.0000628167928PMC5256065 [Google Scholar] [CrossRef] [PubMed]
[6]. Nowak M, Grzeszczak W, Imeglimin: A new antidiabetic drug with potential future in the treatment of patients with type 2 diabetesEndokrynologia Polska 2022 73(2):361-70.10.5603/EP.a2022.001435381095 [Google Scholar] [CrossRef] [PubMed]
[7]. Hallakou-Bozec S, Vial G, Kergoat M, Fouqueray P, Bolze S, Borel AL, Mechanism of action of Imeglimin: A novel therapeutic agent for type 2 diabetesDiabetes Obes Metab 2021 23(3):664-73.10.1111/dom.1427733269554PMC8049051 [Google Scholar] [CrossRef] [PubMed]
[8]. Fouqueray P, Pirags V, Inzucchi SE, Bailey CJ, Schernthaner G, Diamant M, The efficacy and safety of imeglimin as add-on therapy in patients with type 2 diabetes inadequately controlled with metformin monotherapyDiabetes Care 2013 36(3):565-68.10.2337/dc12-045323160726PMC3579350 [Google Scholar] [CrossRef] [PubMed]
[9]. Reilhac C, Dubourg J, Thang C, Grouin JM, Fouqueray P, Watada H, Efficacy and safety of imeglimin add-on to insulin monotherapy in Japanese patients with type 2 diabetes (TIMES 3): A randomized, double-blind, placebo-controlled phase 3 trial with a 36-week open-label extension periodDiabetes Obes Metab 2022 24(5):838-48.10.1111/dom.1464234984815PMC9302620 [Google Scholar] [CrossRef] [PubMed]
[10]. Dubourg J, Fouqueray P, Quinslot D, Grouin JM, Kaku K, Long-term safety and efficacy of imeglimin as monotherapy or in combination with existing antidiabetic agents in Japanese patients with type 2 diabetes (TIMES 2): A 52-week, open-label, multicentre phase 3 trialDiabetes Obes Metab 2022 24(4):609-19.10.1111/dom.1461334866306PMC9305103 [Google Scholar] [CrossRef] [PubMed]
[11]. Saisho Y, β-cell dysfunction: Its critical role in prevention and management of type 2 diabetesWorld J Diabetes 2015 6(1):109-24.10.4239/wjd.v6.i1.10925685282PMC4317303 [Google Scholar] [CrossRef] [PubMed]
[12]. Mezza T, Cinti F, Cefalo CMA, Pontecorvi A, Kulkarni RN, Giaccari A, β-cell fate in human insulin resistance and type 2 diabetes: A perspective on islet plasticityDiabetes 2019 68(6):1121-29.10.2337/db18-085631109941PMC6905483 [Google Scholar] [CrossRef] [PubMed]
[13]. Sena CM, Bento CF, Pereira P, Seiça R, Diabetes mellitus: New challenges and innovative therapiesEPMA J 2010 1(1):138-63.10.1007/s13167-010-0010-923199048PMC3405309 [Google Scholar] [CrossRef] [PubMed]
[14]. Perreault L, Skyler JS, Rosenstock J, Novel therapies with precision mechanisms for type 2 diabetes mellitusNat Rev Endocrinol 2021 17(6):364-77.10.1038/s41574-021-00489-y33948015 [Google Scholar] [CrossRef] [PubMed]
[15]. Lorenzati B, Zucco C, Miglietta S, Lamberti F, Bruno G, Oral hypoglycaemic drugs: Pathophysiological basis of their mechanism of actionPharmaceuticals (Basel) 2010 3(9):3005-20.10.3390/ph309300527713388PMC4034109 [Google Scholar] [CrossRef] [PubMed]
[16]. DeFronzo RA, Eldor R, Abdul-Ghani M, Pathophysiologic approach to therapy in patients with newly diagnosed type 2 diabetesDiabetes Care 2013 36(Suppl 2):S127-S138.10.2337/dcS13-201123882037PMC3920797 [Google Scholar] [CrossRef] [PubMed]
[17]. Nomoto H, Takahashi A, Nakamura A, Kurihara H, Takeuchi J, Nagai S, Add-on imeglimin versus metformin dose escalation regarding glycaemic control in patients with type 2 diabetes treated with a dipeptidyl peptidase-4 inhibitor plus low-dose metformin: Study protocol for a multicenter, prospective, randomized, open-label, parallel-group comparison study (MEGMI study)BMJ Open Diab Res Care 2022 10(6):e00298810.1136/bmjdrc-2022-00298836379585PMC9667996 [Google Scholar] [CrossRef] [PubMed]
[18]. KDIGO- Kidney Disease | Improving Global Outcomes [Internet][cited 2025 Jan 2]. Available from: https://kdigo.org/ [Google Scholar]
[19]. Dubourg J, Fouqueray P, Thang C, Grouin JM, Ueki K, Efficacy and safety of imeglimin monotherapy versus placebo in Japanese patients with type 2 diabetes (TIMES 1): A double-blind, randomized, placebo-controlled, parallel-group, multicenter phase 3 trialDiabetes Care 2021 44(4):952-59.10.2337/dc20-076333574125 [Google Scholar] [CrossRef] [PubMed]
[20]. Fouqueray P, Pirags V, Diamant M, Schernthaner G, Lebovitz HE, Inzucchi SE, The efficacy and safety of imeglimin as add-on therapy in patients with type 2 diabetes inadequately controlled with sitagliptin monotherapyDiabetes Care 2014 37(7):1924-30.10.2337/dc13-234924722500 [Google Scholar] [CrossRef] [PubMed]
[21]. Bouchoucha M, Uzzan B, Cohen R, Metformin and digestive disordersDiabetes Metab 2011 37(2):90-96.10.1016/j.diabet.2010.11.00221236717 [Google Scholar] [CrossRef] [PubMed]
[22]. Oda T, Satoh M, Nagasawa K, Sasaki A, Hasegawa Y, Takebe N, The effects of imeglimin on the daily glycaemic profile evaluated by intermittently scanned continuous glucose monitoring: Retrospective, single-center, observational studyDiabetes Ther 2022 13(9):1635-43.10.1007/s13300-022-01298-w35895275PMC9399333 [Google Scholar] [CrossRef] [PubMed]
[23]. Dubourg J, Ueki K, Grouin JM, Fouqueray P, Efficacy and safety of imeglimin in Japanese patients with type 2 diabetes: A 24-week, randomized, double-blind, placebo-controlled, dose-ranging phase 2b trialDiabetes Obes Metab 2021 23(3):800-10.10.1111/dom.1428533275318PMC7898540 [Google Scholar] [CrossRef] [PubMed]
[24]. Fouqueray P, Leverve X, Fontaine E, Baquié M, Wollheim C, Lebovitz H, Imeglimin-a new oral anti-diabetic that targets the three key defects of type 2 diabetesJ Diabetes Metab 2011 2(4):126Available from: https://www.omicsonline.org/imeglimin-a-new-oral-anti-diabetic-that-targets-the-three-key-defects-of-type-2-diabetes-2155-6156.1000126.php?aid=71410.4172/2155-6156.1000126 [Google Scholar] [CrossRef]